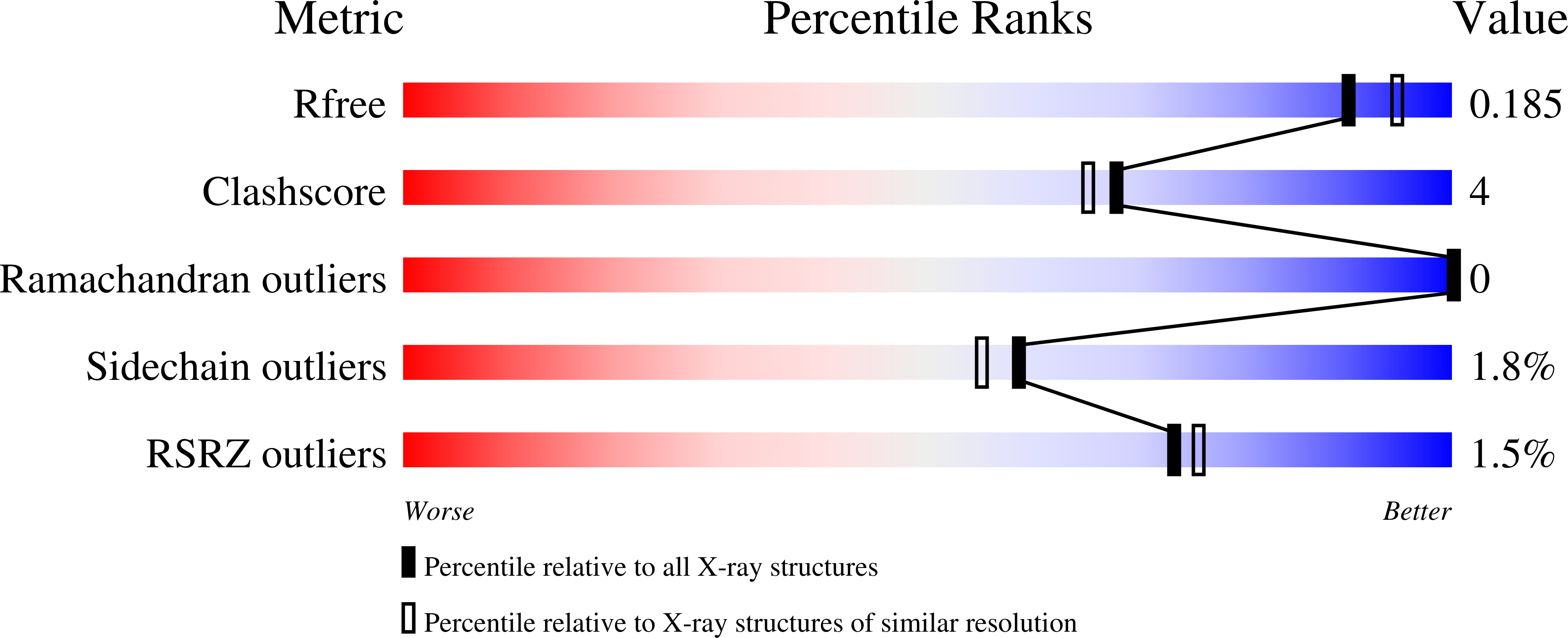
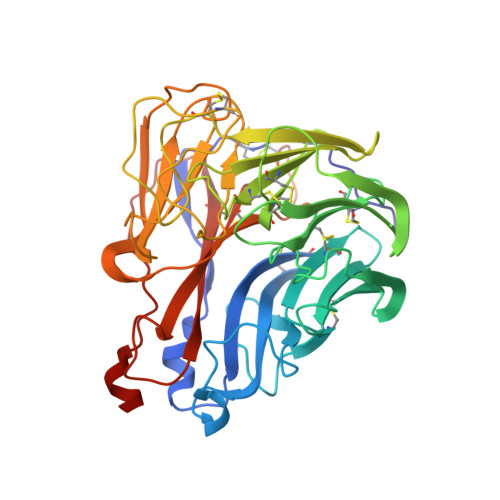
Structural and Functional Analysis of Anti-Influenza Activity of 4-, 7-, 8- and 9-Deoxygenated 2,3-Difluoro- N-acetylneuraminic Acid Derivatives.
McKimm-Breschkin, J.L., Barrett, S., Pilling, P.A., Hader, S., Watts, A.G., Streltsov, V.A.(2018) J Med Chem 61: 1921-1933
- PubMed: 29397718
- DOI: https://doi.org/10.1021/acs.jmedchem.7b01467
- Primary Citation of Related Structures:
5W26, 5W2U, 5W2W, 5W2Y - PubMed Abstract:
Competitive inhibitors of the influenza neuraminidase (NA) were discovered almost 20 years ago, with zanamivir and oseltamivir licensed globally. These compounds are based on a transition state analogue of the sialic acid substrate. We recently showed that 5- N-(acetylamino)-2,3,5-trideoxy-2,3-difluoro-d-erythro-β-l-manno-2-nonulopyranosonic acid (DFSA) and its derivatives are also potent inhibitors of the influenza NA. They are mechanism based inhibitors, forming a covalent bond between the C2 of the sugar ring and Y406 in the NA active site, thus inactivating the enzyme. We have now synthesized a series of deoxygenated DFSA derivatives in order to understand the contribution of each hydroxyl in DFSA to binding and inhibition of the influenza NA. We have investigated their relative efficacy in enzyme assays in vitro, in cell culture, and by X-ray crystallography. We found loss of the 8- and 9-OH had the biggest impact on the affinity of binding and antiviral potency.
Organizational Affiliation:
CSIRO Manufacturing , 343 Royal Parade , Parkville , Victoria 3052 , Australia.