Design of KDM4 Inhibitors with Antiproliferative Effects in Cancer Models.
Chen, Y.K., Bonaldi, T., Cuomo, A., Del Rosario, J.R., Hosfield, D.J., Kanouni, T., Kao, S.C., Lai, C., Lobo, N.A., Matuszkiewicz, J., McGeehan, A., O'Connell, S.M., Shi, L., Stafford, J.A., Stansfield, R.K., Veal, J.M., Weiss, M.S., Yuen, N.Y., Wallace, M.B.(2017) ACS Med Chem Lett 8: 869-874
- PubMed: 28835804
- DOI: https://doi.org/10.1021/acsmedchemlett.7b00220
- Primary Citation of Related Structures:
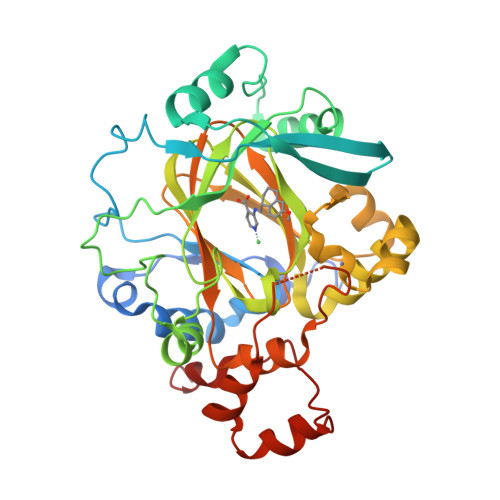
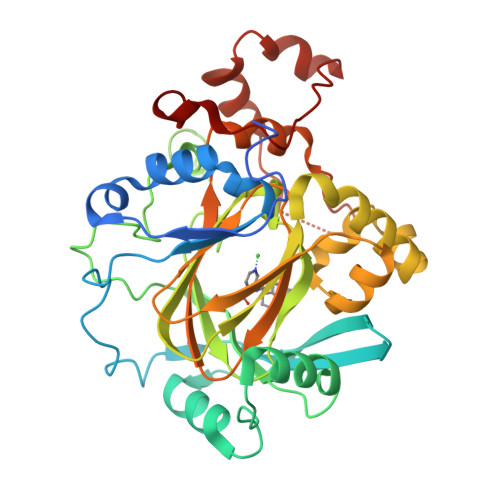
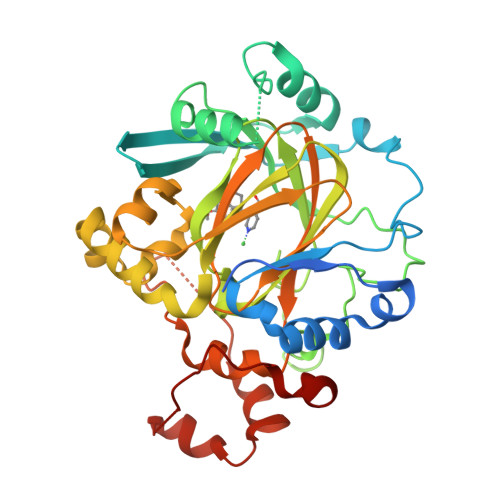
5VGI, 5VMP - PubMed Abstract:
Histone lysine demethylases (KDMs) play a vital role in the regulation of chromatin-related processes. Herein, we describe our discovery of a series of potent KDM4 inhibitors that are both cell permeable and antiproliferative in cancer models. The modulation of histone H3K9me3 and H3K36me3 upon compound treatment was verified by homogeneous time-resolved fluorescence assay and by mass spectroscopy detection. Optimization of the series using structure-based drug design led to compound 6 (QC6352), a potent KDM4 family inhibitor that is efficacious in breast and colon cancer PDX models.
Organizational Affiliation:
Celgene Quanticel Research, 10300 Campus Point Drive, Suite 100, San Diego, California 92121, United States.