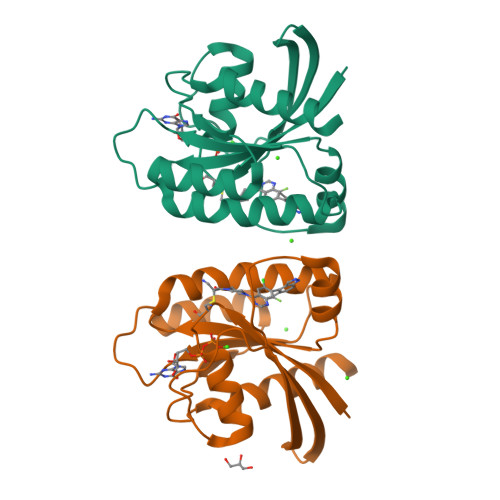
Expanding the Scope of Electrophiles Capable of Targeting K-Ras Oncogenes.
McGregor, L.M., Jenkins, M.L., Kerwin, C., Burke, J.E., Shokat, K.M.(2017) Biochemistry 56: 3178-3183
- PubMed: 28621541
- DOI: https://doi.org/10.1021/acs.biochem.7b00271
- Primary Citation of Related Structures:
5V6S, 5V6V - PubMed Abstract:
There is growing interest in reversible and irreversible covalent inhibitors that target noncatalytic amino acids in target proteins. With a goal of targeting oncogenic K-Ras variants (e.g., G12D) by expanding the types of amino acids that can be targeted by covalent inhibitors, we survey a set of electrophiles for their ability to label carboxylates. We functionalized an optimized ligand for the K-Ras switch II pocket with a set of electrophiles previously reported to react with carboxylates and characterized the ability of these compounds to react with model nucleophiles and oncogenic K-Ras proteins. Here, we report that aziridines and stabilized diazo groups preferentially react with free carboxylates over thiols. Although we did not identify a warhead that potently labels K-Ras G12D, we were able to study the interactions of many electrophiles with K-Ras, as most of the electrophiles rapidly label K-Ras G12C. We characterized the resulting complexes by crystallography, hydrogen/deuterium exchange, and differential scanning fluorimetry. Our results both demonstrate the ability of a noncatalytic cysteine to react with a diverse set of electrophiles and emphasize the importance of proper spatial arrangements between a covalent inhibitor and its intended nucleophile. We hope that these results can expand the range of electrophiles and nucleophiles of use in covalent protein modulation.
Organizational Affiliation:
Howard Hughes Medical Institute and Department of Cellular and Molecular Pharmacology, University of California, San Francisco , San Francisco, California 94158, United States.