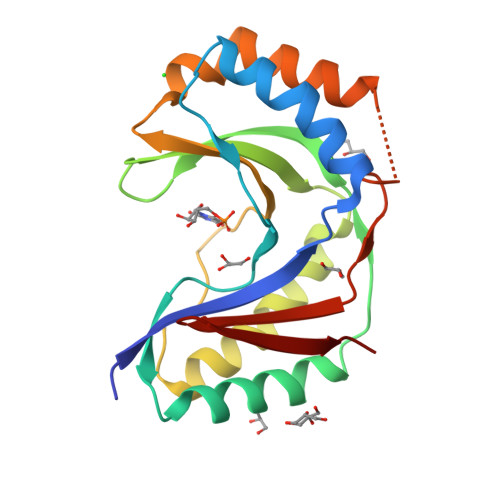
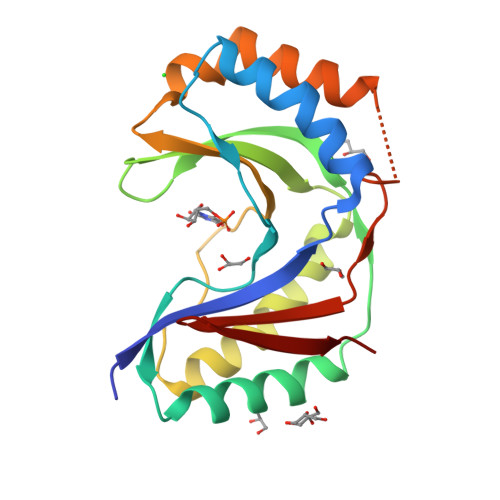
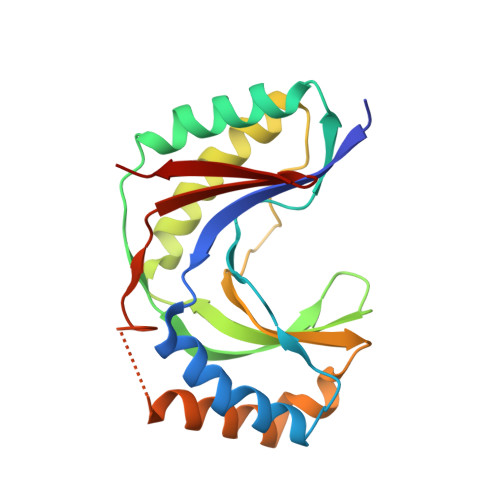
Usb1 controls U6 snRNP assembly through evolutionarily divergent cyclic phosphodiesterase activities.
Didychuk, A.L., Montemayor, E.J., Carrocci, T.J., DeLaitsch, A.T., Lucarelli, S.E., Westler, W.M., Brow, D.A., Hoskins, A.A., Butcher, S.E.(2017) Nat Commun 8: 497-497
- PubMed: 28887445
- DOI: https://doi.org/10.1038/s41467-017-00484-w
- Primary Citation of Related Structures:
5UQJ, 5V1M - PubMed Abstract:
U6 small nuclear ribonucleoprotein (snRNP) biogenesis is essential for spliceosome assembly, but not well understood. Here, we report structures of the U6 RNA processing enzyme Usb1 from yeast and a substrate analog bound complex from humans. Unlike the human ortholog, we show that yeast Usb1 has cyclic phosphodiesterase activity that leaves a terminal 3' phosphate which prevents overprocessing. Usb1 processing of U6 RNA dramatically alters its affinity for cognate RNA-binding proteins. We reconstitute the post-transcriptional assembly of yeast U6 snRNP in vitro, which occurs through a complex series of handoffs involving 10 proteins (Lhp1, Prp24, Usb1 and Lsm2-8) and anti-cooperative interactions between Prp24 and Lhp1. We propose a model for U6 snRNP assembly that explains how evolutionarily divergent and seemingly antagonistic proteins cooperate to protect and chaperone the nascent snRNA during its journey to the spliceosome.The mechanism of U6 small nuclear ribonucleoprotein (snRNP) biogenesis is not well understood. Here the authors characterize the enzymatic activities and structures of yeast and human U6 RNA processing enzyme Usb1, reconstitute post-transcriptional assembly of yeast U6 snRNP in vitro, and propose a model for U6 snRNP assembly.
Organizational Affiliation:
Department of Biochemistry, University of Wisconsin, Madison, Wisconsin, 53706, USA.