HIT family hydrolase from Clostridium thermocellum Cth-393
Habel, J., Tempel, W., Liu, Z.-J., Rose, J., Wang, B.-C.To be published.
Experimental Data Snapshot
Starting Model: experimental
View more details
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Histidine triad (HIT) protein | 147 | Acetivibrio thermocellus DSM 1313 | Mutation(s): 0 Gene Names: Cthe_1369 | 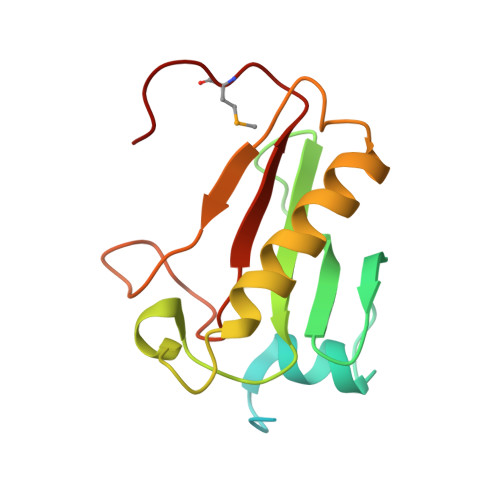 | |
UniProt | |||||
Find proteins for A3DF72 (Acetivibrio thermocellus (strain ATCC 27405 / DSM 1237 / JCM 9322 / NBRC 103400 / NCIMB 10682 / NRRL B-4536 / VPI 7372)) Explore A3DF72 Go to UniProtKB: A3DF72 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | A3DF72 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Ligands 3 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| ADN Query on ADN | BA [auth B] | ADENOSINE C10 H13 N5 O4 OIRDTQYFTABQOQ-KQYNXXCUSA-N |  | ||
| ZN Query on ZN | C [auth A], V [auth B] | ZINC ION Zn PTFCDOFLOPIGGS-UHFFFAOYSA-N |  | ||
| UNX Query on UNX | AA [auth B] CA [auth B] D [auth A] DA [auth B] E [auth A] | UNKNOWN ATOM OR ION X |  | ||
| Modified Residues 1 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Type | Formula | 2D Diagram | Parent |
| MSE Query on MSE | A, B | L-PEPTIDE LINKING | C5 H11 N O2 Se |  | MET |
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 78.804 | α = 90 |
| b = 78.804 | β = 90 |
| c = 114.885 | γ = 90 |
| Software Name | Purpose |
|---|---|
| REFMAC | refinement |
| DENZO | data reduction |
| SCALEPACK | data scaling |
| REFMAC | phasing |