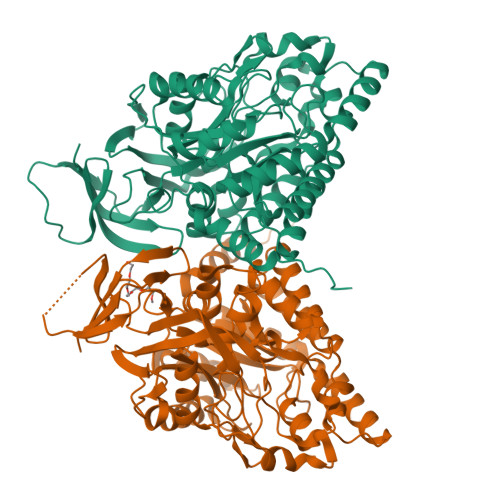
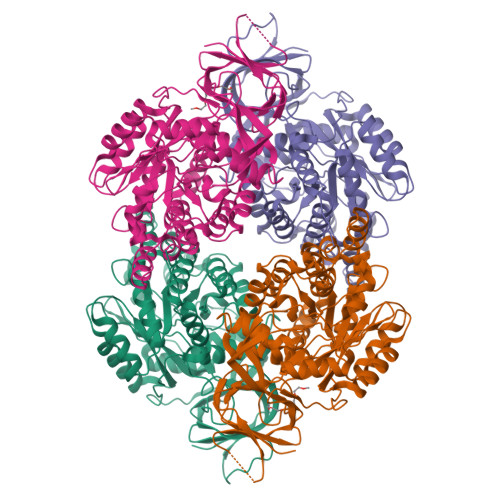
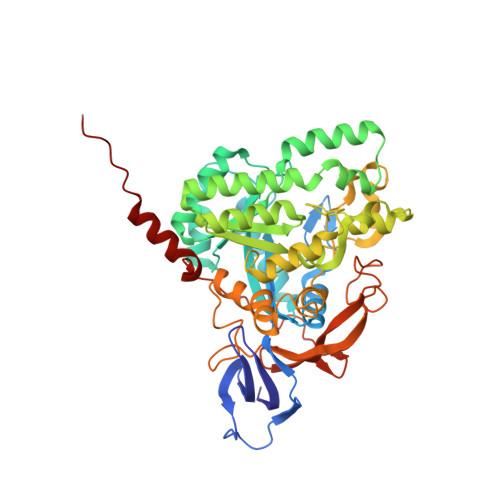
A single structurally conserved SUMOylation site in CRMP2 controls NaV1.7 function.
Dustrude, E.T., Perez-Miller, S., Francois-Moutal, L., Moutal, A., Khanna, M., Khanna, R.(2017) Channels (Austin) 11: 316-328
- PubMed: 28277940
- DOI: https://doi.org/10.1080/19336950.2017.1299838
- Primary Citation of Related Structures:
5UQC - PubMed Abstract:
The neuronal collapsin response mediator protein 2 (CRMP2) undergoes several posttranslational modifications that codify its functions. Most recently, CRMP2 SUMOylation (addition of small ubiquitin like modifier (SUMO)) was identified as a key regulatory step within a modification program that codes for CRMP2 interaction with, and trafficking of, voltage-gated sodium channel NaV1.7. In this paper, we illustrate the utility of combining sequence alignment within protein families with structural analysis to identify, from several putative SUMOylation sites, those that are most likely to be biologically relevant. Co-opting this principle to CRMP2, we demonstrate that, of 3 sites predicted to be SUMOylated in CRMP2, only the lysine 374 site is a SUMOylation client. A reduction in NaV1.7 currents was the corollary of the loss of CRMP2 SUMOylation at this site. A 1.78-Å-resolution crystal structure of mouse CRMP2 was solved using X-ray crystallography, revealing lysine 374 as buried within the CRMP2 tetramer interface but exposed in the monomer. Since CRMP2 SUMOylation is dependent on phosphorylation, we postulate that this state forces CRMP2 toward a monomer, exposing the SUMO site and consequently, resulting in constitutive regulation of NaV1.7.
Organizational Affiliation:
a Department of Pharmacology, College of Medicine , University of Arizona , Tucson , AZ , USA.