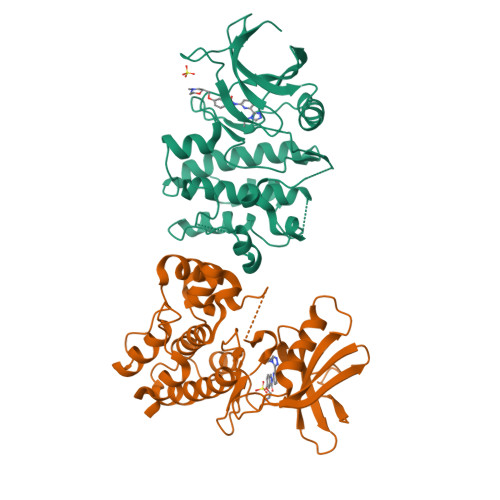
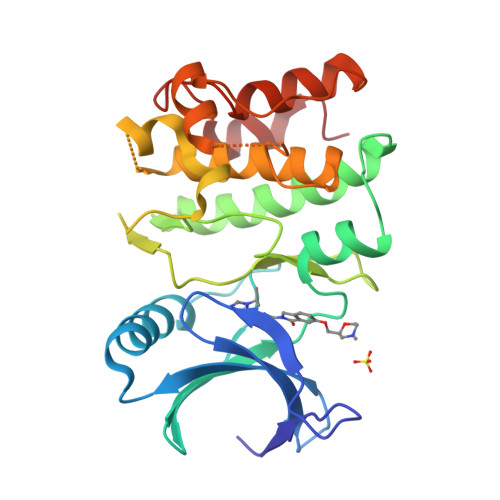
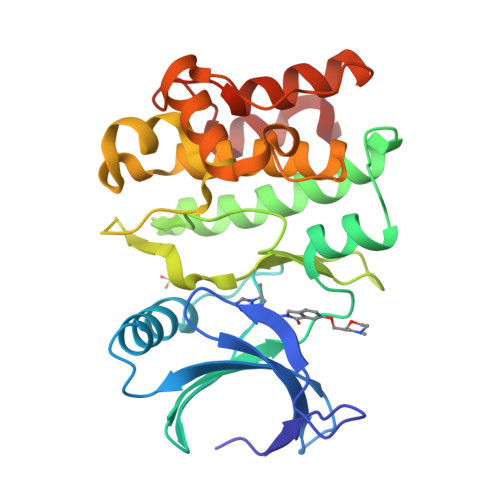
Structure-Based Design of ASK1 Inhibitors as Potential Agents for Heart Failure.
Lanier, M., Pickens, J., Bigi, S.V., Bradshaw-Pierce, E.L., Chambers, A., Cheruvallath, Z.S., Cole, D., Dougan, D.R., Ermolieff, J., Gibson, T., Halkowycz, P., Hirokawa, A., Ivetac, A., Miura, J., Nunez, E., Sabat, M., Tyhonas, J., Wang, H., Wang, X., Swann, S.(2017) ACS Med Chem Lett 8: 316-320
- PubMed: 28337323
- DOI: https://doi.org/10.1021/acsmedchemlett.6b00481
- Primary Citation of Related Structures:
5UOR, 5UOX, 5UP3 - PubMed Abstract:
Apoptosis signal-regulating kinase 1 (ASK1/MAP3K) is a mitogen-activated protein kinase family member shown to contribute to acute ischemia/reperfusion injury. Using structure-based drug design, deconstruction, and reoptimization of a known ASK1 inhibitor, a lead compound was identified. This compound displayed robust MAP3K pathway inhibition and reduction of infarct size in an isolated perfused heart model of cardiac injury.
Organizational Affiliation:
Departments of Medicinal Chemistry, Drug Metabolism Pharmacokinetics, Structural Biology, and Discovery Biology, Gastrointestinal Drug Discovery Unit, Takeda Pharmaceuticals , 10410 Science Center Drive, San Diego, California 92121, United States.