A potent small-molecule inhibitor of the DCN1-UBC12 interaction that selectively blocks cullin 3 neddylation.
Zhou, H., Lu, J., Liu, L., Bernard, D., Yang, C.Y., Fernandez-Salas, E., Chinnaswamy, K., Layton, S., Stuckey, J., Yu, Q., Zhou, W., Pan, Z., Sun, Y., Wang, S.(2017) Nat Commun 8: 1150-1150
- PubMed: 29074978
- DOI: https://doi.org/10.1038/s41467-017-01243-7
- Primary Citation of Related Structures:
5UFI - PubMed Abstract:
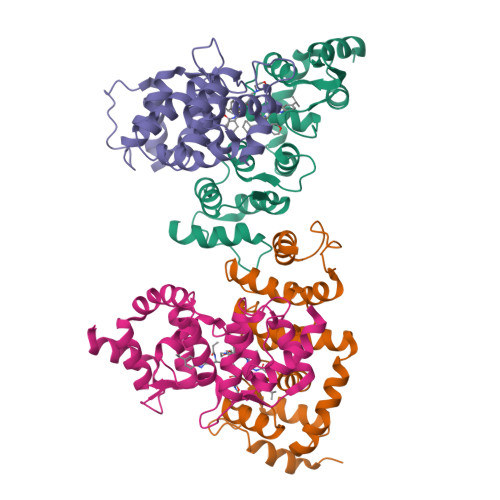
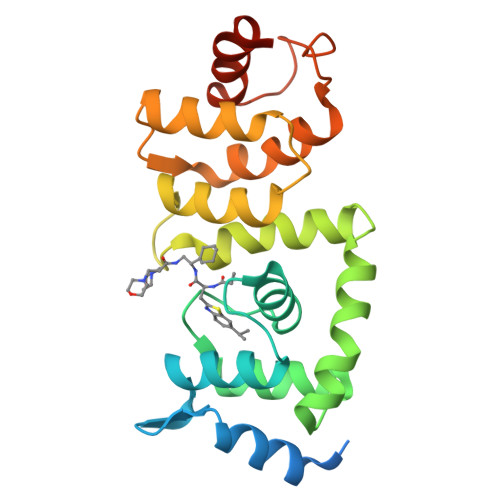
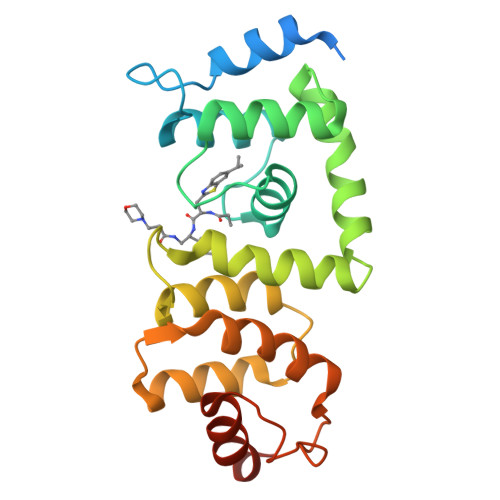
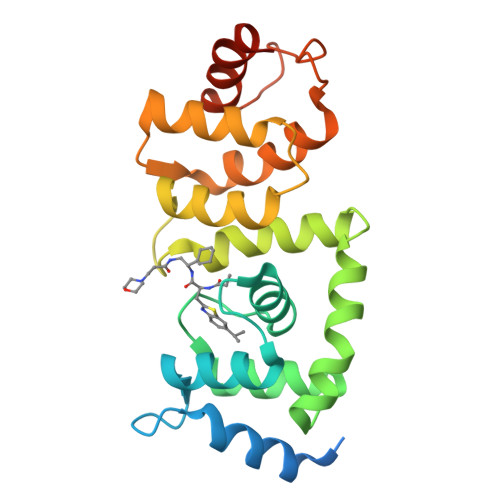
The Cullin-RING E3 ubiquitin ligases (CRLs) regulate homeostasis of ~20% of cellular proteins and their activation require neddylation of their cullin subunit. Cullin neddylation is modulated by a scaffolding DCN protein through interactions with both the cullin protein and an E2 enzyme such as UBC12. Here we report the development of DI-591 as a high-affinity, cell-permeable small-molecule inhibitor of the DCN1-UBC12 interaction. DI-591 binds to purified recombinant human DCN1 and DCN2 proteins with K i values of 10-12 nM, and disrupts the DCN1-UBC12 interaction in cells. Treatment with DI-591 selectively converts cellular cullin 3 into an un-neddylated inactive form with no or minimum effect on other cullin members. Our data firmly establish a previously unrecognized specific role of the DCN1-UBC12 interaction for cellular neddylation of cullin 3. DI-591 is an excellent probe compound to investigate the role of the cullin 3 CRL ligase in biological processes and human diseases.
Organizational Affiliation:
Department of Internal Medicine, University of Michigan, Ann Arbor, Michigan, 48109, USA.