Preferential induction of cross-group influenza A hemagglutinin stem-specific memory B cells after H7N9 immunization in humans.
Andrews, S.F., Joyce, M.G., Chambers, M.J., Gillespie, R.A., Kanekiyo, M., Leung, K., Yang, E.S., Tsybovsky, Y., Wheatley, A.K., Crank, M.C., Boyington, J.C., Prabhakaran, M.S., Narpala, S.R., Chen, X., Bailer, R.T., Chen, G., Coates, E., Kwong, P.D., Koup, R.A., Mascola, J.R., Graham, B.S., Ledgerwood, J.E., McDermott, A.B.(2017) Sci Immunol 2
- PubMed: 28783708
- DOI: https://doi.org/10.1126/sciimmunol.aan2676
- Primary Citation of Related Structures:
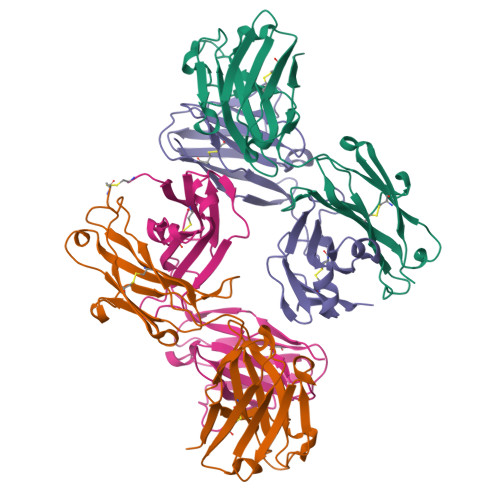
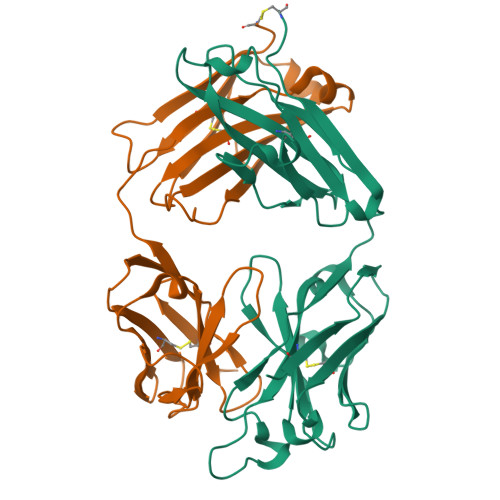
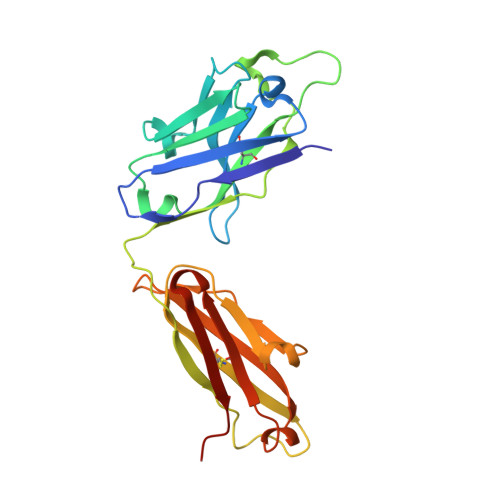
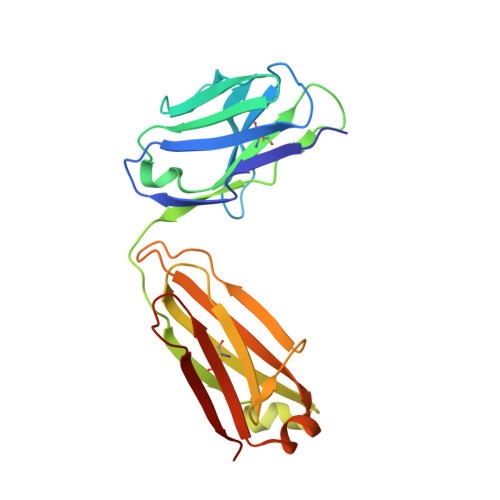
5TY6, 5U4R, 5WCA, 5WCC, 5WCD - PubMed Abstract:
Antigenic drift and shift of influenza strains underscore the need for broadly protective influenza vaccines. One strategy is to design immunogens that elicit B cell responses against conserved epitopes on the hemagglutinin (HA) stem. To better understand the elicitation of HA stem-targeted B cells to group 1 and group 2 influenza subtypes, we compared the memory B cell response to group 2 H7N9 and group 1 H5N1 vaccines in humans. Upon H7N9 vaccination, almost half of the HA stem-specific response recognized the group 1 and group 2 subtypes, whereas the response to H5N1 was largely group 1-specific. Immunoglobulin repertoire analysis of HA-specific B cells indicated that the H7N9 and H5N1 vaccines induced genetically similar cross-group HA stem-binding B cells, albeit at a much higher frequency upon H7N9 vaccination. These data suggest that a group 2-based stem immunogen could prove more effective than a group 1 immunogen at eliciting broad cross-group protection in humans.
Organizational Affiliation:
Vaccine Research Center, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD 20892, USA. adrian.mcdermott@nih.gov sarah.andrews2@nih.gov.