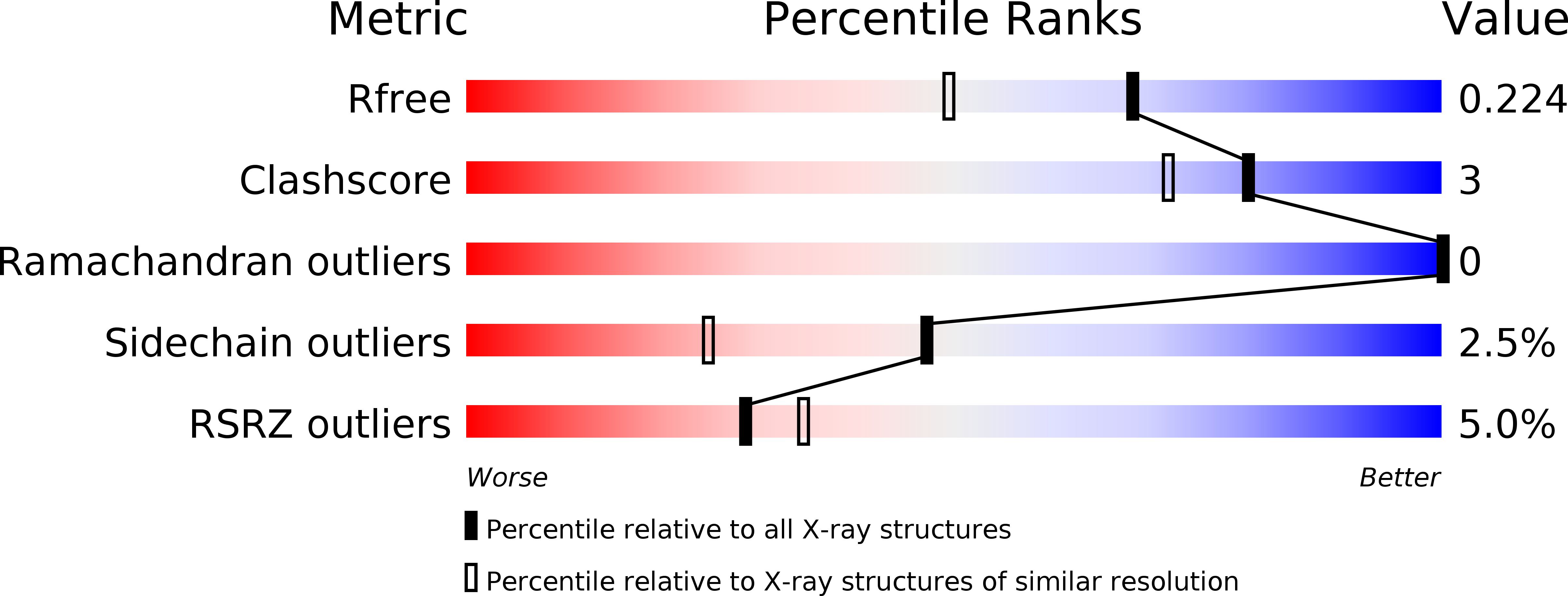
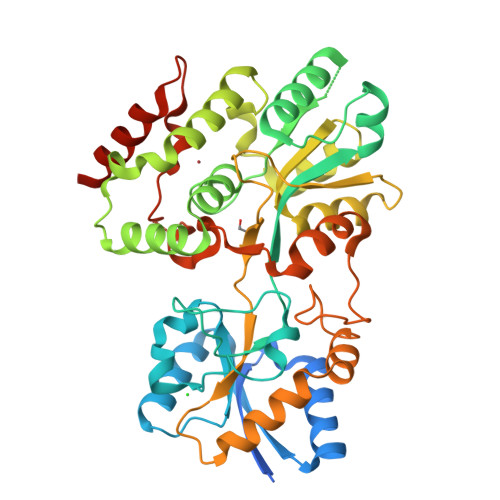
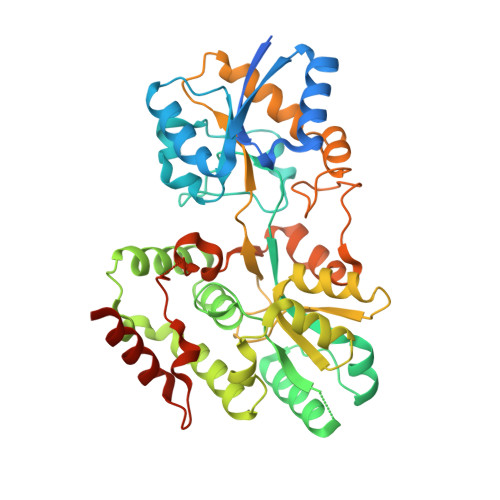
Functional clues from the crystal structure of an orphan periplasmic ligand-binding protein from Treponema pallidum.
Brautigam, C.A., Deka, R.K., Liu, W.Z., Tomchick, D.R., Norgard, M.V.(2017) Protein Sci 26: 847-856
- PubMed: 28168761
- DOI: https://doi.org/10.1002/pro.3133
- Primary Citation of Related Structures:
5U2P - PubMed Abstract:
The spirochete Treponema pallidum is the causative agent of syphilis, a sexually transmitted infection of major global importance. Other closely related subspecies of Treponema also are the etiological agents of the endemic treponematoses, such as yaws, pinta, and bejel. The inability of T. pallidum and its close relatives to be cultured in vitro has prompted efforts to characterize T. pallidum's proteins structurally and biophysically, particularly those potentially relevant to treponemal membrane biology, with the goal of possibly revealing the functions of those proteins. This report describes the structure of the treponemal protein Tp0737; this polypeptide has a fold characteristic of a class of periplasmic ligand-binding proteins associated with ABC-type transporters. Although no ligand for the protein was observed in electron-density maps, and thus the nature of the native ligand remains obscure, the structural data described herein provide a foundation for further efforts to elucidate the ligand and thus the function of this protein in T. pallidum.
Organizational Affiliation:
Department of Biophysics, The University of Texas Southwestern Medical Center, Dallas, Texas, 75390.