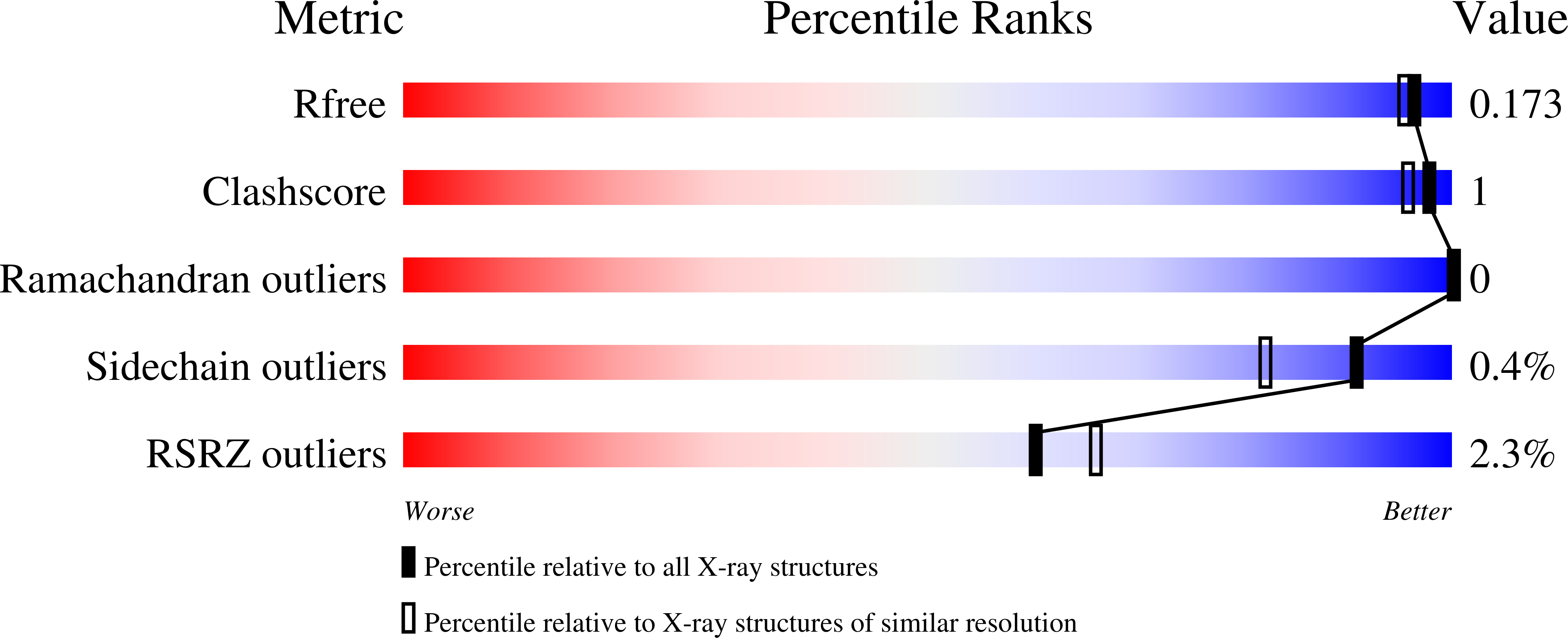
Identification of a New Zinc Binding Chemotype by Fragment Screening.
Chrysanthopoulos, P.K., Mujumdar, P., Woods, L.A., Dolezal, O., Ren, B., Peat, T.S., Poulsen, S.A.(2017) J Med Chem 60: 7333-7349
- PubMed: 28817930
- DOI: https://doi.org/10.1021/acs.jmedchem.7b00606
- Primary Citation of Related Structures:
5TXY, 5TY1, 5TY8, 5TY9, 5TYA, 5U0D, 5U0E, 5U0F, 5U0G, 5VGY - PubMed Abstract:
The discovery of a new zinc binding chemotype from screening a nonbiased fragment library is reported. Using the orthogonal fragment screening methods of native state mass spectrometry and surface plasmon resonance a 3-unsubstituted 2,4-oxazolidinedione fragment was found to have low micromolar binding affinity to the zinc metalloenzyme carbonic anhydrase II (CA II). This affinity approached that of fragment sized primary benzenesulfonamides, the classical zinc binding group found in most CA II inhibitors. Protein X-ray crystallography established that 3-unsubstituted 2,4-oxazolidinediones bound to CA II via an interaction of the acidic ring nitrogen with the CA II active site zinc, as well as two hydrogen bonds between the oxazolidinedione ring oxygen and the CA II protein backbone. Furthermore, 3-unsubstituted 2,4-oxazolidinediones appear to be a viable starting point for the development of an alternative class of CA inhibitor, wherein the medicinal chemistry pedigree of primary sulfonamides has dominated for several decades.
Organizational Affiliation:
Griffith University , Griffith Institute for Drug Discovery, Nathan, Brisbane, Queensland 4111, Australia.