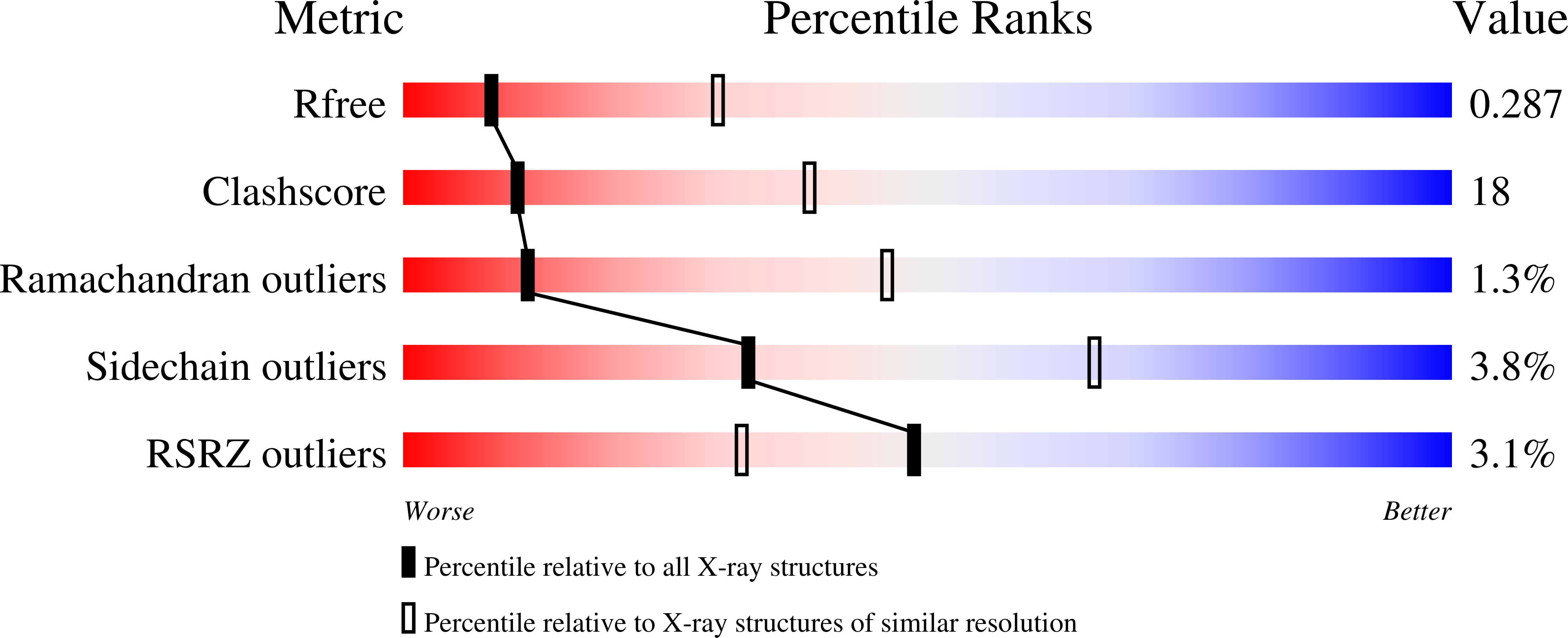
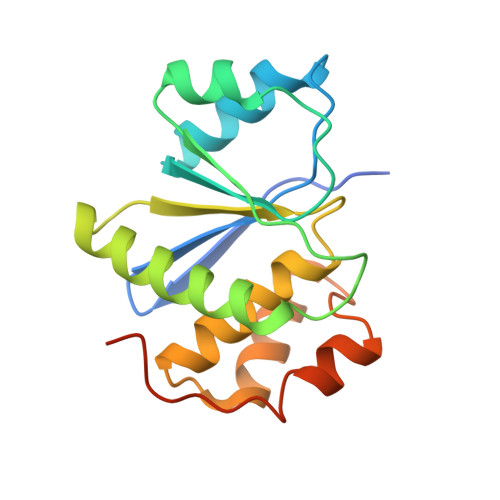
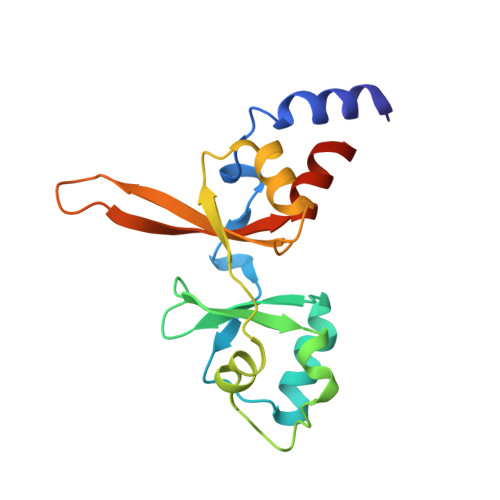
PRL3 phosphatase active site is required for binding the putative magnesium transporter CNNM3.
Zhang, H., Kozlov, G., Li, X., Wu, H., Gulerez, I., Gehring, K.(2017) Sci Rep 7: 48-48
- PubMed: 28246390
- DOI: https://doi.org/10.1038/s41598-017-00147-2
- Primary Citation of Related Structures:
5K23, 5K24, 5K25, 5TSR - PubMed Abstract:
The phosphatases of regenerating liver (PRLs) are involved in tumorigenesis and metastatic cancer yet their cellular function remains unclear. Recent reports have shown PRL phosphatases bind tightly to the CNNM family of membrane proteins to regulate magnesium efflux. Here, we characterize the interactions between the CBS-pair (Bateman) domain of CNNM3 and either PRL2 or PRL3 using X-ray crystallography, isothermal titration calorimetry, and activity assays. We report four new crystal structures of PRL proteins bound to the CNNM3 CBS-pair domain that reveal the effects of cysteine disulphide formation and nucleotide binding on complex formation. We use comprehensive mutagenesis of the PRL3 catalytic site to quantify the importance of different PRL amino acids, including cysteine 104, leucine 108, and arginine 110, for CNNM binding and phosphatase activity. We show the PRL3 R138E mutant is selectively deficient in CNNM3 binding with the potential to distinguish between the downstream effects of phosphatase and CNNM-binding activities in vivo. Through a novel activity assay, we show that PRL3 has magnesium-sensitive phosphatase activity with ATP and other nucleotides. Our results identify a strong correlation between phosphatase activity and CNNM binding and support the contention that PRL function as pseudophosphatases regulated by chemical modifications of their catalytic cysteine.
Organizational Affiliation:
Department of Biochemistry, McGill University, Montreal, Quebec, Canada.