Molecular basis of the evolution of alternative tyrosine biosynthetic routes in plants.
Schenck, C.A., Holland, C.K., Schneider, M.R., Men, Y., Lee, S.G., Jez, J.M., Maeda, H.A.(2017) Nat Chem Biol 13: 1029-1035
- PubMed: 28671678
- DOI: https://doi.org/10.1038/nchembio.2414
- Primary Citation of Related Structures:
5T95, 5T9E, 5T9F, 5WHX - PubMed Abstract:
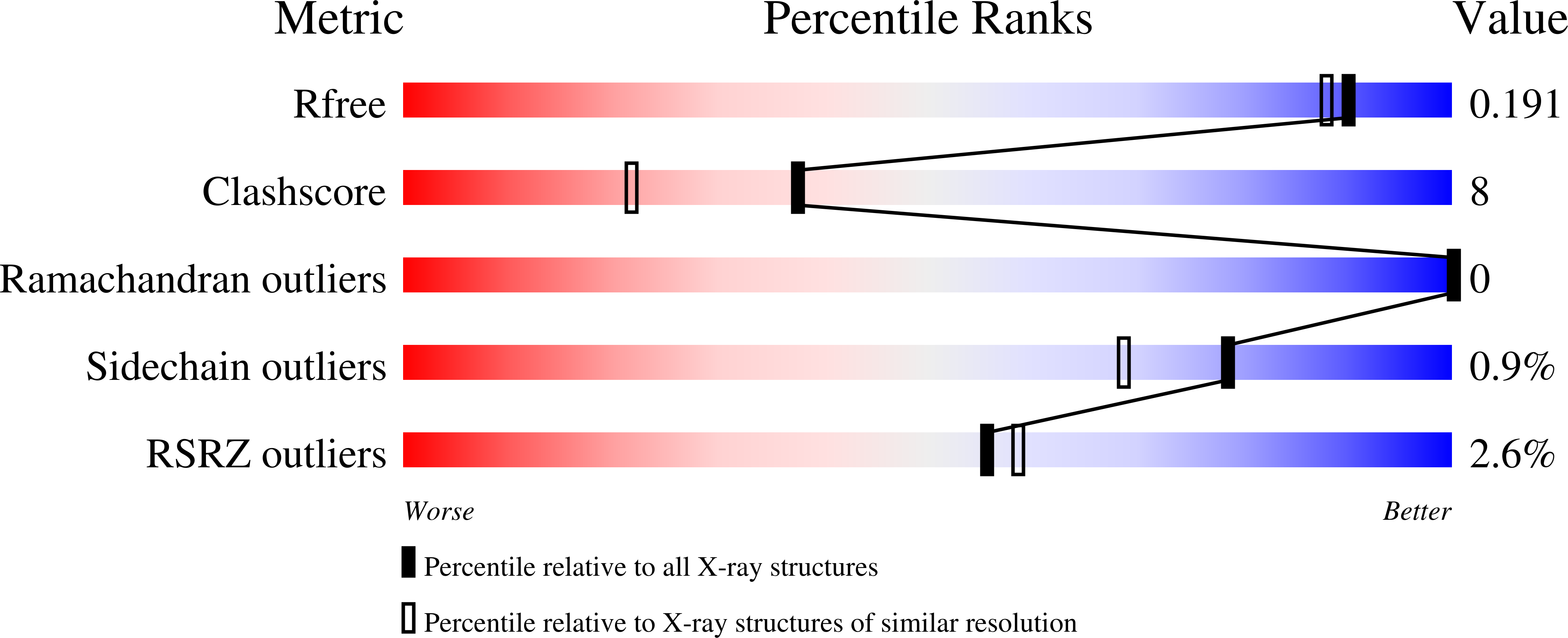
L-Tyrosine (Tyr) is essential for protein synthesis and is a precursor of numerous specialized metabolites crucial for plant and human health. Tyr can be synthesized via two alternative routes by different key regulatory TyrA family enzymes, prephenate dehydrogenase (PDH, also known as TyrA p ) or arogenate dehydrogenase (ADH, also known as TyrA a ), representing a unique divergence of primary metabolic pathways. The molecular foundation underlying the evolution of these alternative Tyr pathways is currently unknown. Here we characterized recently diverged plant PDH and ADH enzymes, obtained the X-ray crystal structure of soybean PDH, and identified a single amino acid residue that defines TyrA substrate specificity and regulation. Structures of mutated PDHs co-crystallized with Tyr indicate that substitutions of Asn222 confer ADH activity and Tyr sensitivity. Reciprocal mutagenesis of the corresponding residue in divergent plant ADHs further introduced PDH activity and relaxed Tyr sensitivity, highlighting the critical role of this residue in TyrA substrate specificity that underlies the evolution of alternative Tyr biosynthetic pathways in plants.
Organizational Affiliation:
Department of Botany, University of Wisconsin-Madison, Madison, Wisconsin, USA.