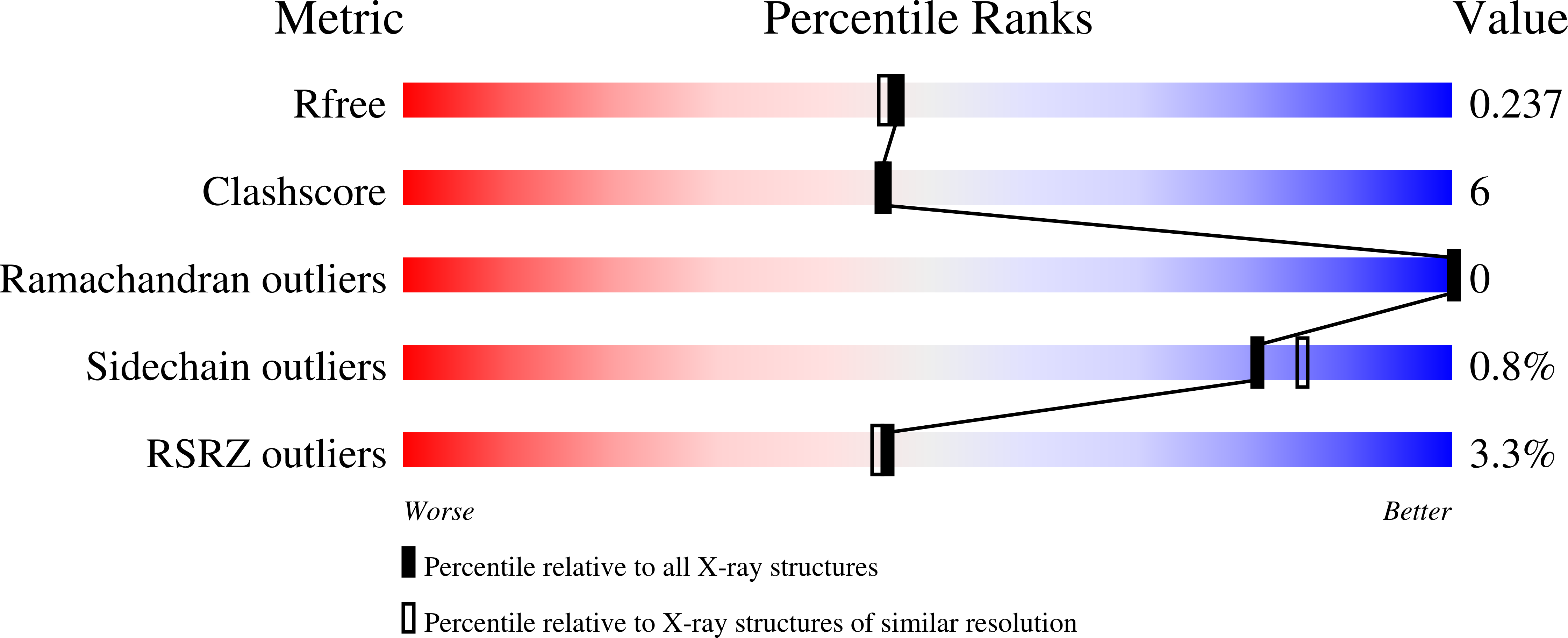
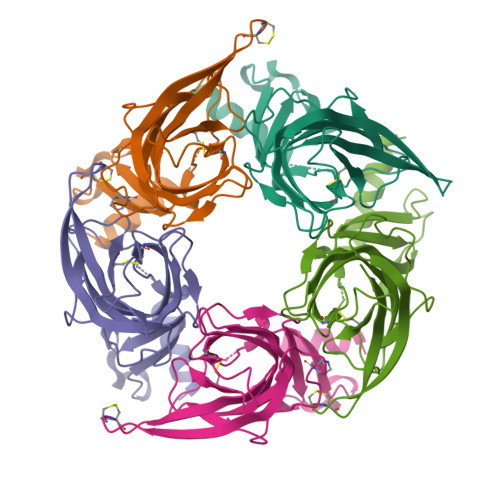
Crystal structure of a chimeric acetylcholine binding protein from Aplysia californica (Ac-AChBP) containing loop C from the human alpha 3 nicotinic acetylcholine receptor in complex with Cytisine
Bobango, J., Wu, J., Talley, I.T., Talley, T.T.To be published.