Molecular Mechanism for Isoform-Selective Inhibition of Acyl Protein Thioesterases 1 and 2 (APT1 and APT2).
Won, S.J., Davda, D., Labby, K.J., Hwang, S.Y., Pricer, R., Majmudar, J.D., Armacost, K.A., Rodriguez, L.A., Rodriguez, C.L., Chong, F.S., Torossian, K.A., Palakurthi, J., Hur, E.S., Meagher, J.L., Brooks, C.L., Stuckey, J.A., Martin, B.R.(2016) ACS Chem Biol 11: 3374-3382
- PubMed: 27748579
- DOI: https://doi.org/10.1021/acschembio.6b00720
- Primary Citation of Related Structures:
5SYM, 5SYN - PubMed Abstract:
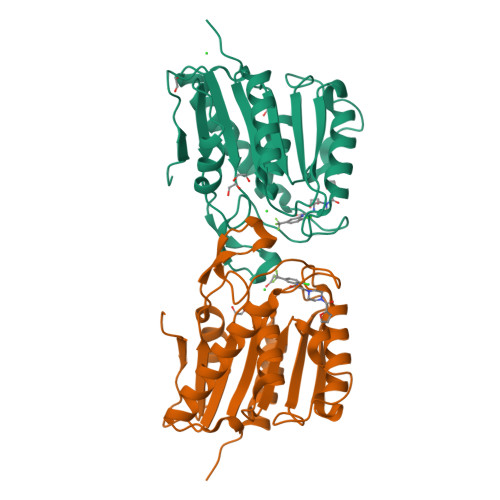
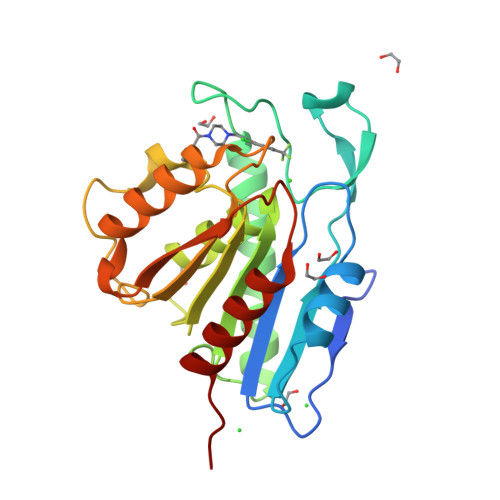
Post-translational S-palmitoylation directs the trafficking and membrane localization of hundreds of cellular proteins, often involving a coordinated palmitoylation cycle that requires both protein acyl transferases (PATs) and acyl protein thioesterases (APTs) to actively redistribute S-palmitoylated proteins toward different cellular membrane compartments. This process is necessary for the trafficking and oncogenic signaling of S-palmitoylated Ras isoforms, and potentially many peripheral membrane proteins. The depalmitoylating enzymes APT1 and APT2 are separately conserved in all vertebrates, suggesting unique functional roles for each enzyme. The recent discovery of the APT isoform-selective inhibitors ML348 and ML349 has opened new possibilities to probe the function of each enzyme, yet it remains unclear how each inhibitor achieves orthogonal inhibition. Herein, we report the high-resolution structure of human APT2 in complex with ML349 (1.64 Å), as well as the complementary structure of human APT1 bound to ML348 (1.55 Å). Although the overall peptide backbone structures are nearly identical, each inhibitor adopts a distinct conformation within each active site. In APT1, the trifluoromethyl group of ML348 is positioned above the catalytic triad, but in APT2, the sulfonyl group of ML349 forms hydrogen bonds with active site resident waters to indirectly engage the catalytic triad and oxyanion hole. Reciprocal mutagenesis and activity profiling revealed several differing residues surrounding the active site that serve as critical gatekeepers for isoform accessibility and dynamics. Structural and biochemical analysis suggests the inhibitors occupy a putative acyl-binding region, establishing the mechanism for isoform-specific inhibition, hydrolysis of acyl substrates, and structural orthogonality important for future probe development.
Organizational Affiliation:
Program in Chemical Biology, ‡Department of Chemistry, §Department of Biophysics, and ∥Life Sciences Institute, University of Michigan , 930 N. University Ave., Ann Arbor, Michigan 48109, United States.