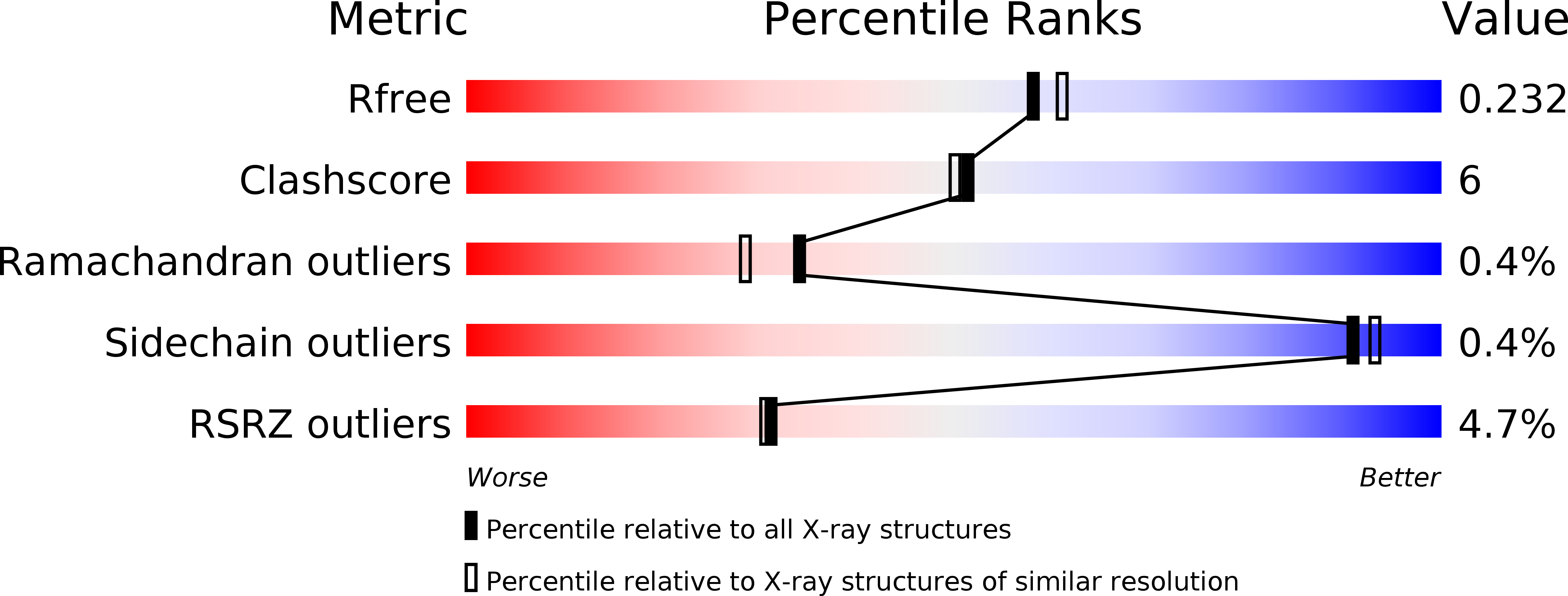
Structures of monomeric and oligomeric forms of theToxoplasma gondiiperforin-like protein 1.
Ni, T., Williams, S.I., Rezelj, S., Anderluh, G., Harlos, K., Stansfeld, P.J., Gilbert, R.J.C.(2018) Sci Adv 4: eaaq0762-eaaq0762
- PubMed: 29750191
- DOI: https://doi.org/10.1126/sciadv.aaq0762
- Primary Citation of Related Structures:
5OUO, 5OUP, 5OUQ, 5OWN - PubMed Abstract:
Toxoplasma and Plasmodium are the parasitic agents of toxoplasmosis and malaria, respectively, and use perforin-like proteins (PLPs) to invade host organisms and complete their life cycles. The Toxoplasma gondii PLP1 ( Tg PLP1) is required for efficient exit from parasitophorous vacuoles in which proliferation occurs. We report structures of the membrane attack complex/perforin (MACPF) and Apicomplexan PLP C-terminal β-pleated sheet (APCβ) domains of Tg PLP1. The MACPF domain forms hexameric assemblies, with ring and helix geometries, and the APCβ domain has a novel β-prism fold joined to the MACPF domain by a short linker. Molecular dynamics simulations suggest that the helical MACPF oligomer preserves a biologically important interface, whereas the APCβ domain binds preferentially through a hydrophobic loop to membrane phosphatidylethanolamine, enhanced by the additional presence of inositol phosphate lipids. This mode of membrane binding is supported by site-directed mutagenesis data from a liposome-based assay. Together, these structural and biophysical findings provide insights into the molecular mechanism of membrane targeting by Tg PLP1.
Organizational Affiliation:
Division of Structural Biology, Wellcome Trust Centre for Human Genetics, University of Oxford, Roosevelt Drive, Oxford OX3 7BN, UK.