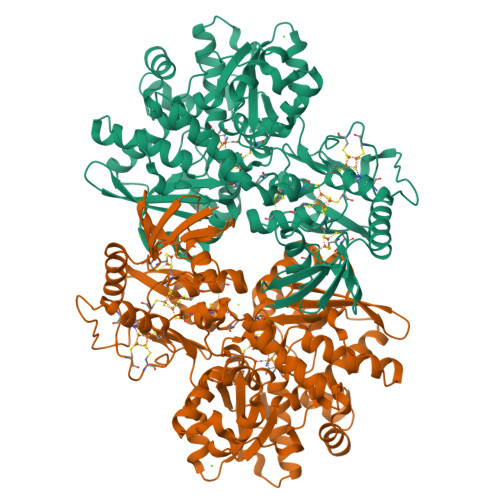
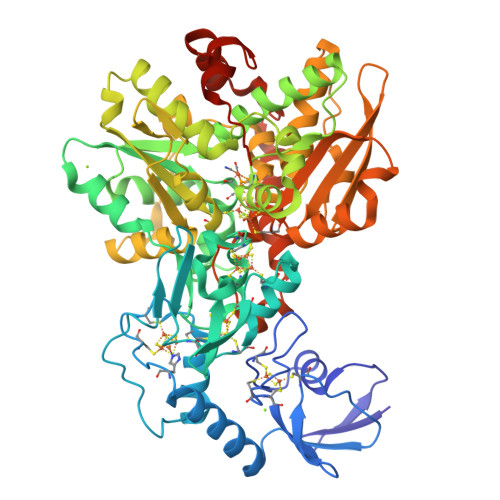
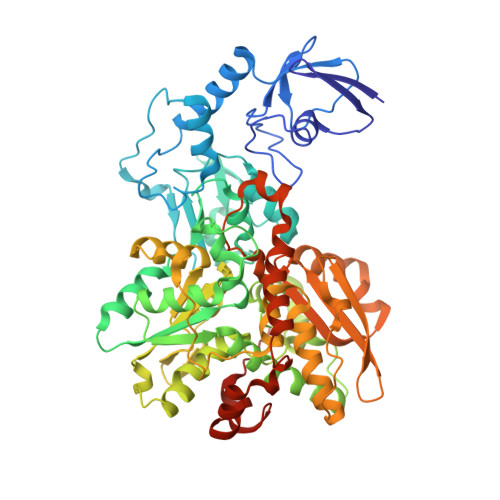
Chalcogenide substitution in the [2Fe] cluster of [FeFe]-hydrogenases conserves high enzymatic activity.
Kertess, L., Wittkamp, F., Sommer, C., Esselborn, J., Rudiger, O., Reijerse, E.J., Hofmann, E., Lubitz, W., Winkler, M., Happe, T., Apfel, U.P.(2017) Dalton Trans 46: 16947-16958
- PubMed: 29177350
- DOI: https://doi.org/10.1039/c7dt03785f
- Primary Citation of Related Structures:
5OEF - PubMed Abstract:
[FeFe]-Hydrogenases efficiently catalyze the uptake and evolution of H 2 due to the presence of an inorganic [6Fe-6S]-cofactor (H-cluster). This cofactor is comprised of a [4Fe-4S] cluster coupled to a unique [2Fe] cluster where the catalytic turnover of H 2 /H + takes place. We herein report on the synthesis of a selenium substituted [2Fe] cluster [Fe 2 {μ(SeCH 2 ) 2 NH}(CO) 4 (CN) 2 ] 2- (ADSe) and its successful in vitro integration into the native protein scaffold of [FeFe]-hydrogenases HydA1 from Chlamydomonas reinhardtii and CpI from Clostridium pasteurianum yielding fully active enzymes (HydA1-ADSe and CpI-ADSe). FT-IR spectroscopy and X-ray structure analysis confirmed the presence of structurally intact ADSe at the active site. Electrochemical assays reveal that the selenium containing enzymes are more biased towards hydrogen production than their native counterparts. In contrast to previous chalcogenide exchange studies, the S to Se exchange herein is not based on a simple reconstitution approach using ionic cluster constituents but on the in vitro maturation with a pre-synthesized selenium-containing [2Fe] mimic. The combination of biological and chemical methods allowed for the creation of a novel [FeFe]-hydrogenase with a [2Fe2Se]-active site which confers individual catalytic features.
Organizational Affiliation:
Ruhr-Universität Bochum, Lehrstuhl für Biochemie der Pflanzen, AG Photobiotechnologie, Universitätsstraße 150, 44801 Bochum, Germany. Thomas.Happe@rub.de.