Inhibitors against Fungal Cell Wall Remodeling Enzymes.
Delso, I., Valero-Gonzalez, J., Gomollon-Bel, F., Castro-Lopez, J., Fang, W., Navratilova, I., van Aalten, D.M.F., Tejero, T., Merino, P., Hurtado-Guerrero, R.(2018) ChemMedChem 13: 128-132
- PubMed: 29164827
- DOI: https://doi.org/10.1002/cmdc.201700720
- Primary Citation of Related Structures:
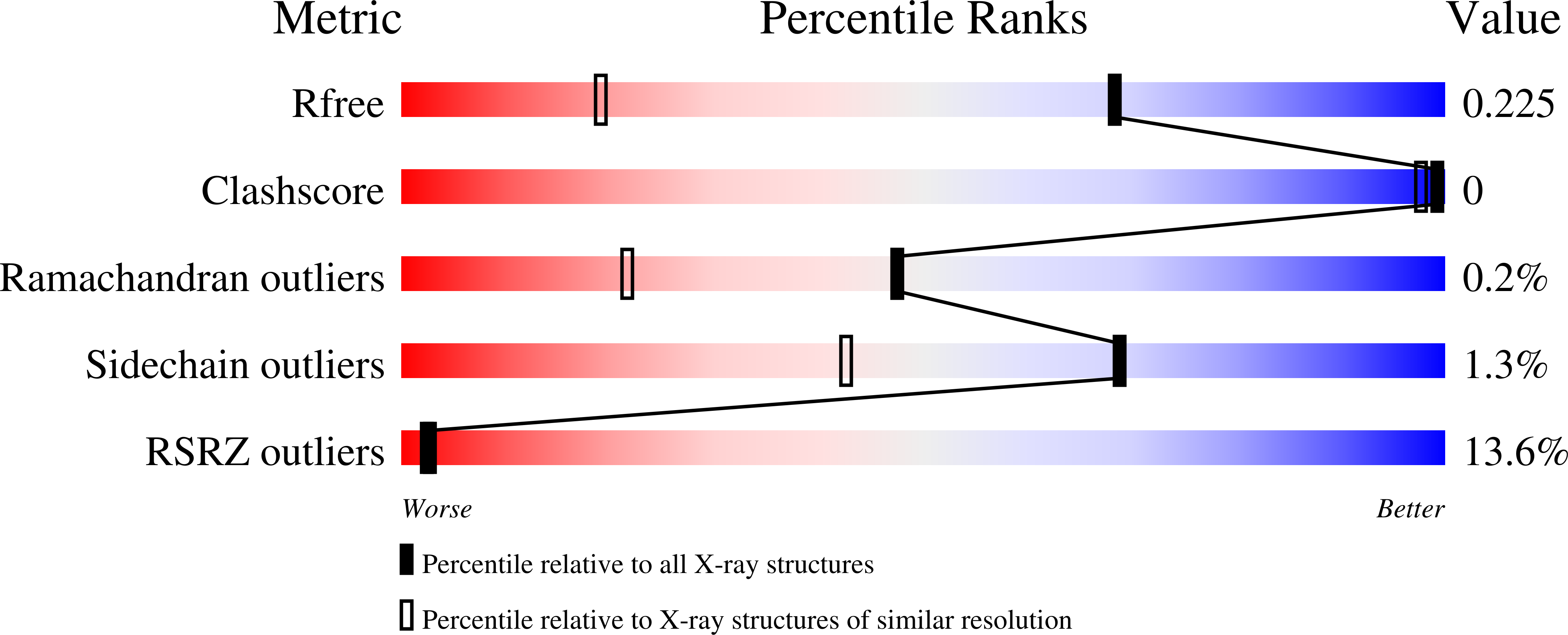
5O9O, 5O9P, 5O9Q, 5O9R, 5O9Y, 5OA2, 5OA6 - PubMed Abstract:
Fungal β-1,3-glucan glucanosyltransferases are glucan-remodeling enzymes that play important roles in cell wall integrity, and are essential for the viability of pathogenic fungi and yeasts. As such, they are considered possible drug targets, although inhibitors of this class of enzymes have not yet been reported. Herein we report a multidisciplinary approach based on a structure-guided design using a highly conserved transglycosylase from Sacharomyces cerevisiae, that leads to carbohydrate derivatives with high affinity for Aspergillus fumigatus Gel4. We demonstrate by X-ray crystallography that the compounds bind in the active site of Gas2/Gel4 and interact with the catalytic machinery. The topological analysis of noncovalent interactions demonstrates that the combination of a triazole with positively charged aromatic moieties are important for optimal interactions with Gas2/Gel4 through unusual pyridinium cation-π and face-to-face π-π interactions. The lead compound is capable of inhibiting AfGel4 with an IC 50 value of 42 μm.
Organizational Affiliation:
Instituto de Síntesis Química y Catálisis Homogénea (ISQCH), Universidad de Zaragoza, CSIC, 50009, Zaragoza, Aragón, Spain.