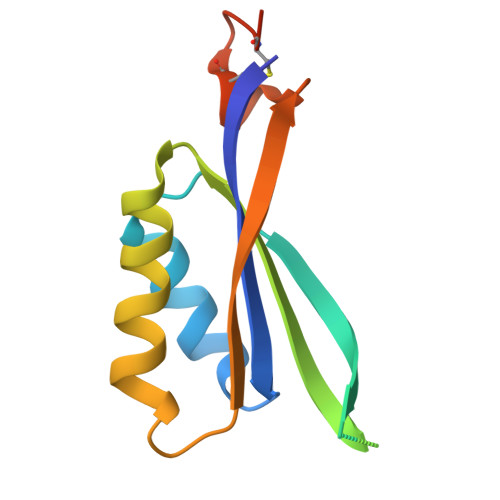
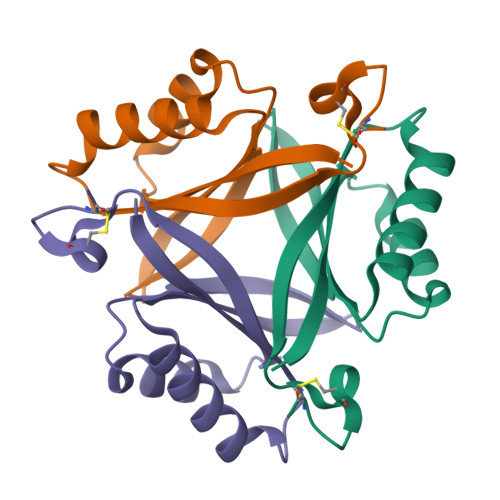
PII-like signaling protein SbtB links cAMP sensing with cyanobacterial inorganic carbon response.
Selim, K.A., Haase, F., Hartmann, M.D., Hagemann, M., Forchhammer, K.(2018) Proc Natl Acad Sci U S A 115: E4861-E4869
- PubMed: 29735650
- DOI: https://doi.org/10.1073/pnas.1803790115
- Primary Citation of Related Structures:
5O3P, 5O3Q, 5O3R, 5O3S - PubMed Abstract:
Cyanobacteria are phototrophic prokaryotes that evolved oxygenic photosynthesis ∼2.7 billion y ago and are presently responsible for ∼10% of total global photosynthetic production. To cope with the evolutionary pressure of dropping ambient CO 2 concentrations, they evolved a CO 2 -concentrating mechanism (CCM) to augment intracellular inorganic carbon (C i ) levels for efficient CO 2 fixation. However, how cyanobacteria sense the fluctuation in C i is poorly understood. Here we present biochemical, structural, and physiological insights into SbtB, a unique P II -like signaling protein, which provides new insights into C i sensing. SbtB is highly conserved in cyanobacteria and is coexpressed with CCM genes. The SbtB protein from the cyanobacterium Synechocystis sp. PCC 6803 bound a variety of adenosine nucleotides, including the second messenger cAMP. Cocrystal structures unraveled the individual binding modes of trimeric SbtB with AMP and cAMP. The nucleotide-binding pocket is located between the subunit clefts of SbtB, perfectly matching the structure of canonical P II proteins. This clearly indicates that proteins of the P II superfamily arose from a common ancestor, whose structurally conserved nucleotide-binding pocket has evolved to sense different adenyl nucleotides for various signaling functions. Moreover, we provide physiological and biochemical evidence for the involvement of SbtB in C i acclimation. Collectively, our results suggest that SbtB acts as a C i sensor protein via cAMP binding, highlighting an evolutionarily conserved role for cAMP in signaling the cellular carbon status.
Organizational Affiliation:
Organismic Interactions Department, Interfaculty Institute for Microbiology and Infection Medicine, Tübingen University, 72076 Tübingen, Germany; khaled.selim@uni-tuebingen.de karl.forchhammer@uni-tuebingen.de.