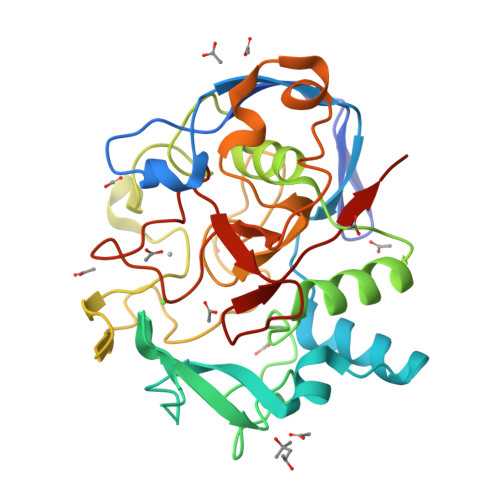
Structural Basis for Copper-Oxygen Mediated C-H Bond Activation by the Formylglycine-Generating Enzyme.
Meury, M., Knop, M., Seebeck, F.P.(2017) Angew Chem Int Ed Engl 56: 8115-8119
- PubMed: 28544744
- DOI: https://doi.org/10.1002/anie.201702901
- Primary Citation of Related Structures:
5NXL, 5NYY - PubMed Abstract:
The formylglycine-generating enzyme (FGE) is a unique copper protein that catalyzes oxygen-dependent C-H activation. We describe 1.66 Å- and 1.28 Å-resolution crystal structures of FGE from Thermomonospora curvata in complex with either Ag I or Cd II providing definitive evidence for a high-affinity metal-binding site in this enzyme. The structures reveal a bis-cysteine linear coordination of the monovalent metal, and tetrahedral coordination of the bivalent metal. Similar coordination changes may occur in the active enzyme as a result of Cu I/II redox cycling. Complexation of copper atoms by two cysteine residues is common among copper-trafficking proteins, but is unprecedented for redox-active copper enzymes or synthetic copper catalysts.
Organizational Affiliation:
Department for Chemistry, University of Basel, St. Johanns-Ring 19, 4056, Basel, Switzerland.