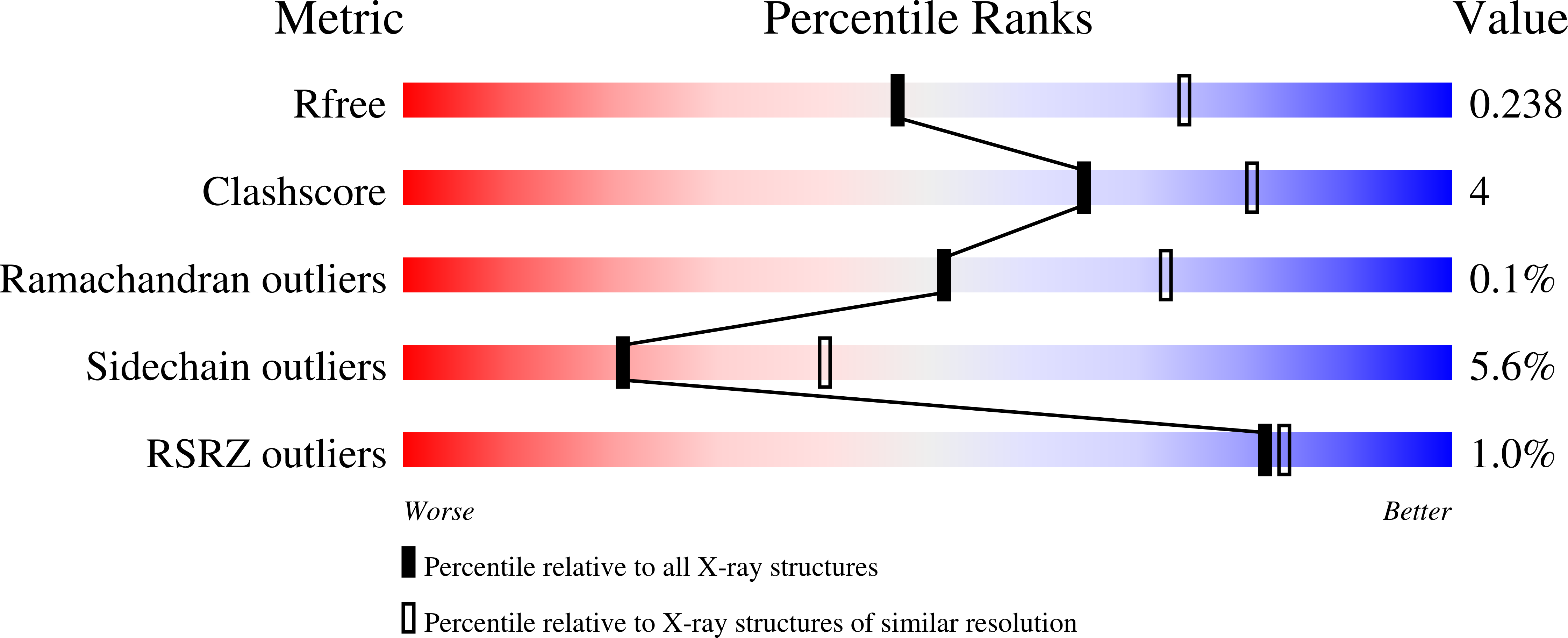
Xenon for tunnelling analysis of the efflux pump component OprN.
Ntsogo Enguene, Y.V., Phan, G., Garnier, C., Ducruix, A., Prange, T., Broutin, I.(2017) PLoS One 12: e0184045-e0184045
- PubMed: 28886086
- DOI: https://doi.org/10.1371/journal.pone.0184045
- Primary Citation of Related Structures:
5IUY, 5NSW - PubMed Abstract:
Tripartite efflux pumps are among the main actors responsible for antibiotics resistance in Gram-negative bacteria. In the last two decades, structural studies gave crucial information about the assembly interfaces and the mechanistic motions. Thus rigidifying the assembly seems to be an interesting way to hamper the drug efflux. In this context, xenon is a suitable probe for checking whether small ligands could act as conformational lockers by targeting hydrophobic cavities. Here we focus on OprN, the outer membrane channel of the MexEF efflux pump from Pseudomonas aeruginosa. After exposing OprN crystals to xenon gas pressure, 14 binding sites were observed using X-ray crystallography. These binding sites were unambiguously characterized in hydrophobic cavities of OprN. The major site is observed in the sensitive iris-like region gating the channel at the periplasmic side, built by the three key-residues Leu 405, Asp 109, and Arg 412. This arrangement defines along the tunnel axis a strong hydrophobic/polar gradient able to enhance the passive efflux mechanism of OprN. The other xenon atoms reveal strategic hydrophobic regions of the channel scaffold to target, with the aim to freeze the dynamic movements responsible of the open/close conformational equilibrium in OprN.
Organizational Affiliation:
Laboratoire de Cristallographie et RMN Biologiques (UMR 8015, CNRS), Faculté de Pharmacie, Université Paris Descartes, USPC, Paris, France.