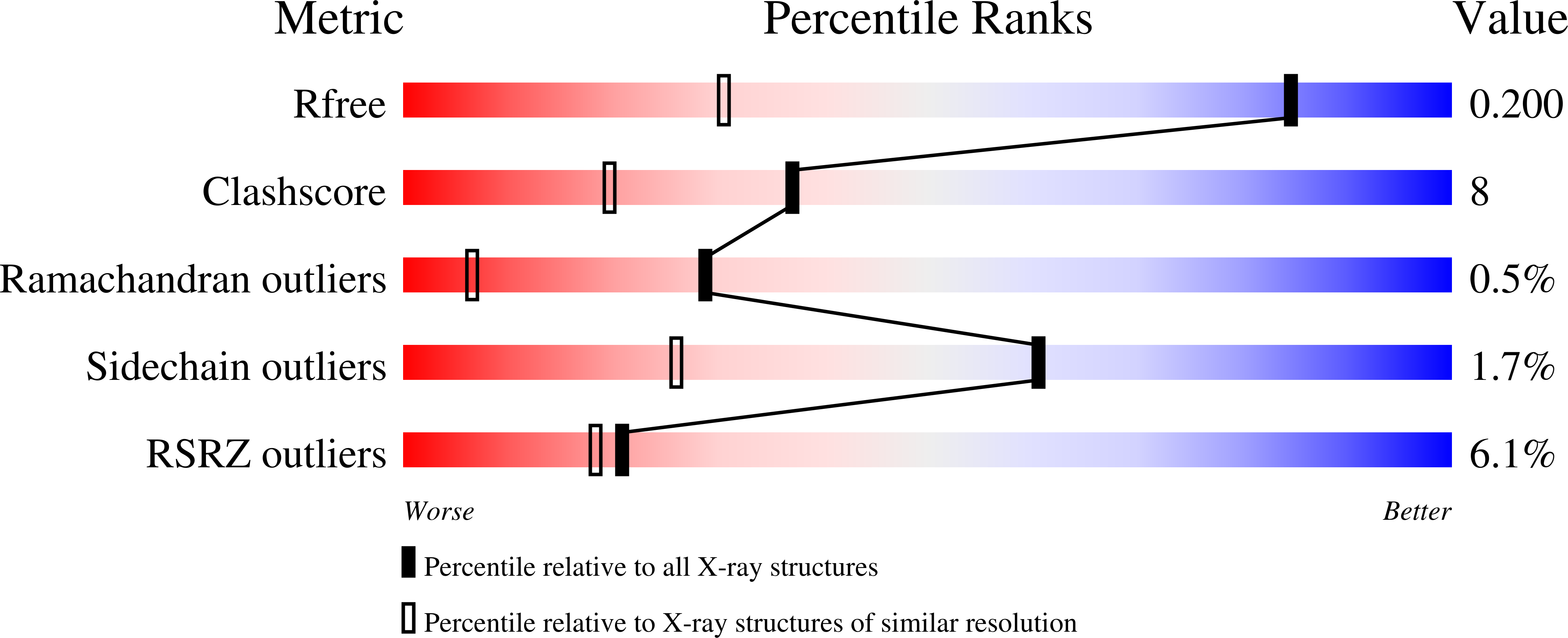
Burkholderia pseudomallei d-alanine-d-alanine ligase; detailed characterisation and assessment of a potential antibiotic drug target.
Diaz-Saez, L., Torrie, L.S., McElroy, S.P., Gray, D., Hunter, W.N.(2019) FEBS J
- PubMed: 31260169
- DOI: https://doi.org/10.1111/febs.14976
- Primary Citation of Related Structures:
5NRH, 5NRI - PubMed Abstract:
Burkholderia pseudomallei is a serious, difficult to treat Gram-negative pathogen and an increase in the occurrence of drug-resistant strains has been detected. We have directed efforts to identify and to evaluate potential drug targets relevant to treatment of infection by B. pseudomallei. We have selected and characterised the essential enzyme d-alanine-d-alanine ligase (BpDdl), required for the ATP-assisted biosynthesis of a peptidoglycan precursor. A recombinant supply of protein supported high-resolution crystallographic and biophysical studies with ligands (AMP and AMP+d-Ala-d-Ala), and comparisons with orthologues enzymes suggest a ligand-induced conformational change occurring that might be relevant to the catalytic cycle. The detailed biochemical characterisation of the enzyme, development and optimisation of ligand binding assays supported the search for novel inhibitors by screening of selected compound libraries. In a similar manner to that observed previously in other studies, we note a paucity of hits that are worth follow-up and then in combination with a computational analysis of the active site, we conclude that this ligase represents a difficult target for drug discovery. Nevertheless, our reagents, protocols and data can underpin future efforts exploiting more diverse chemical libraries and structure-based approaches.
Organizational Affiliation:
Division of Biological Chemistry and Drug Discovery, School of Life Sciences, University of Dundee, UK.