Low pH-induced conformational change and dimerization of sortilin triggers endocytosed ligand release.
Leloup, N., Lossl, P., Meijer, D.H., Brennich, M., Heck, A.J.R., Thies-Weesie, D.M.E., Janssen, B.J.C.(2017) Nat Commun 8: 1708-1708
- PubMed: 29167428
- DOI: https://doi.org/10.1038/s41467-017-01485-5
- Primary Citation of Related Structures:
5NMR, 5NMT, 5NNI, 5NNJ - PubMed Abstract:
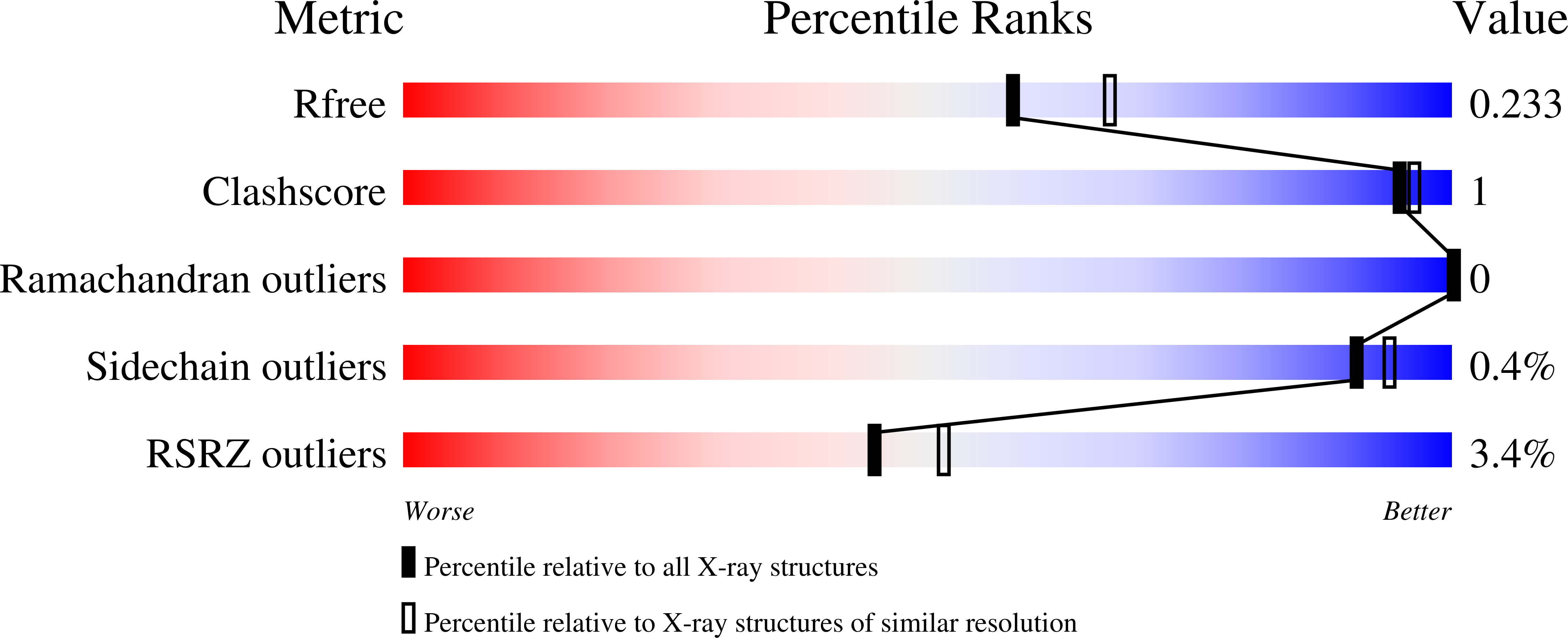
Low pH-induced ligand release and receptor recycling are important steps for endocytosis. The transmembrane protein sortilin, a β-propeller containing endocytosis receptor, internalizes a diverse set of ligands with roles in cell differentiation and homeostasis. The molecular mechanisms of pH-mediated ligand release and sortilin recycling are unresolved. Here we present crystal structures that show the sortilin luminal segment (s-sortilin) undergoes a conformational change and dimerizes at low pH. The conformational change, within all three sortilin luminal domains, provides an altered surface and the dimers sterically shield a large interface while bringing the two s-sortilin C-termini into close proximity. Biophysical and cell-based assays show that members of two different ligand families, (pro)neurotrophins and neurotensin, preferentially bind the sortilin monomer. This indicates that sortilin dimerization and conformational change discharges ligands and triggers recycling. More generally, this work may reveal a double mechanism for low pH-induced ligand release by endocytosis receptors.
Organizational Affiliation:
Crystal and Structural Chemistry, Bijvoet Center for Biomolecular Research, Faculty of Science, Utrecht University, 3584 CH, Utrecht, The Netherlands.