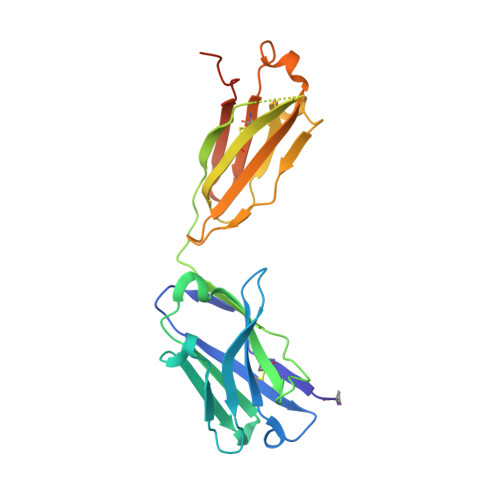
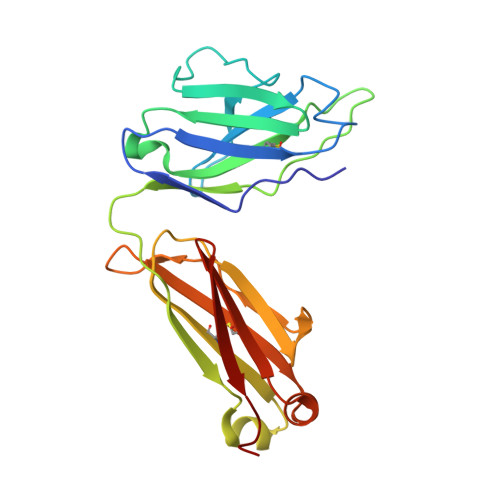
Blockade of activin type II receptors with a dual anti-ActRIIA/IIB antibody is critical to promote maximal skeletal muscle hypertrophy.
Morvan, F., Rondeau, J.M., Zou, C., Minetti, G., Scheufler, C., Scharenberg, M., Jacobi, C., Brebbia, P., Ritter, V., Toussaint, G., Koelbing, C., Leber, X., Schilb, A., Witte, F., Lehmann, S., Koch, E., Geisse, S., Glass, D.J., Lach-Trifilieff, E.(2017) Proc Natl Acad Sci U S A 114: 12448-12453
- PubMed: 29109273
- DOI: https://doi.org/10.1073/pnas.1707925114
- Primary Citation of Related Structures:
5NGV, 5NH3, 5NHR, 5NHW - PubMed Abstract:
The TGF-β family ligands myostatin, GDF11, and activins are negative regulators of skeletal muscle mass, which have been reported to primarily signal via the ActRIIB receptor on skeletal muscle and thereby induce muscle wasting described as cachexia. Use of a soluble ActRIIB-Fc "trap," to block myostatin pathway signaling in normal or cachectic mice leads to hypertrophy or prevention of muscle loss, perhaps suggesting that the ActRIIB receptor is primarily responsible for muscle growth regulation. Genetic evidence demonstrates however that both ActRIIB- and ActRIIA-deficient mice display a hypertrophic phenotype. Here, we describe the mode of action of bimagrumab (BYM338), as a human dual-specific anti-ActRIIA/ActRIIB antibody, at the molecular and cellular levels. As shown by X-ray analysis, bimagrumab binds to both ActRIIA and ActRIIB ligand binding domains in a competitive manner at the critical myostatin/activin binding site, hence preventing signal transduction through either ActRII. Myostatin and the activins are capable of binding to both ActRIIA and ActRIIB, with different affinities. However, blockade of either single receptor through the use of specific anti-ActRIIA or anti-ActRIIB antibodies achieves only a partial signaling blockade upon myostatin or activin A stimulation, and this leads to only a small increase in muscle mass. Complete neutralization and maximal anabolic response are achieved only by simultaneous blockade of both receptors. These findings demonstrate the importance of ActRIIA in addition to ActRIIB in mediating myostatin and activin signaling and highlight the need for blocking both receptors to achieve a strong functional benefit.
Organizational Affiliation:
MusculoSkeletal Diseases, Novartis Institutes for Biomedical Research, 4002 Basel, Switzerland.