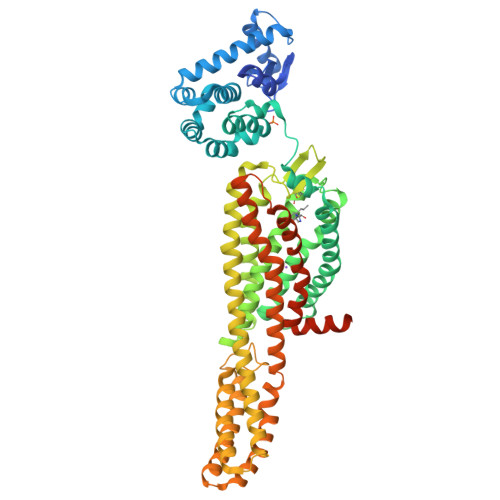
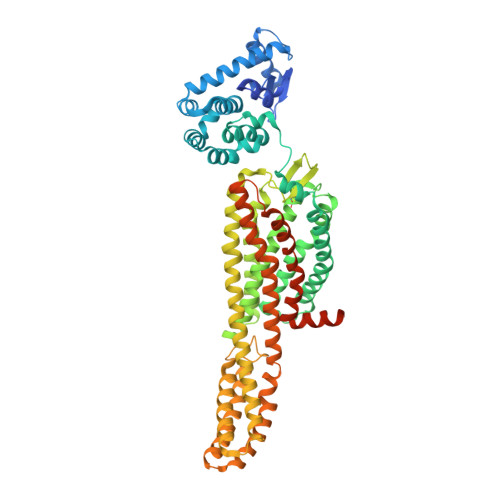
Structural insight into allosteric modulation of protease-activated receptor 2.
Cheng, R.K.Y., Fiez-Vandal, C., Schlenker, O., Edman, K., Aggeler, B., Brown, D.G., Brown, G.A., Cooke, R.M., Dumelin, C.E., Dore, A.S., Geschwindner, S., Grebner, C., Hermansson, N.O., Jazayeri, A., Johansson, P., Leong, L., Prihandoko, R., Rappas, M., Soutter, H., Snijder, A., Sundstrom, L., Tehan, B., Thornton, P., Troast, D., Wiggin, G., Zhukov, A., Marshall, F.H., Dekker, N.(2017) Nature 545: 112-115
- PubMed: 28445455
- DOI: https://doi.org/10.1038/nature22309
- Primary Citation of Related Structures:
5NDD, 5NDZ, 5NJ6 - PubMed Abstract:
Protease-activated receptors (PARs) are a family of G-protein-coupled receptors (GPCRs) that are irreversibly activated by proteolytic cleavage of the N terminus, which unmasks a tethered peptide ligand that binds and activates the transmembrane receptor domain, eliciting a cellular cascade in response to inflammatory signals and other stimuli. PARs are implicated in a wide range of diseases, such as cancer and inflammation. PARs have been the subject of major pharmaceutical research efforts but the discovery of small-molecule antagonists that effectively bind them has proved challenging. The only marketed drug targeting a PAR is vorapaxar, a selective antagonist of PAR1 used to prevent thrombosis. The structure of PAR1 in complex with vorapaxar has been reported previously. Despite sequence homology across the PAR isoforms, discovery of PAR2 antagonists has been less successful, although GB88 has been described as a weak antagonist. Here we report crystal structures of PAR2 in complex with two distinct antagonists and a blocking antibody. The antagonist AZ8838 binds in a fully occluded pocket near the extracellular surface. Functional and binding studies reveal that AZ8838 exhibits slow binding kinetics, which is an attractive feature for a PAR2 antagonist competing against a tethered ligand. Antagonist AZ3451 binds to a remote allosteric site outside the helical bundle. We propose that antagonist binding prevents structural rearrangements required for receptor activation and signalling. We also show that a blocking antibody antigen-binding fragment binds to the extracellular surface of PAR2, preventing access of the tethered ligand to the peptide-binding site. These structures provide a basis for the development of selective PAR2 antagonists for a range of therapeutic uses.
Organizational Affiliation:
Heptares Therapeutics Ltd, BioPark, Broadwater Road, Welwyn Garden City, Hertfordshire AL7 3AX, UK.