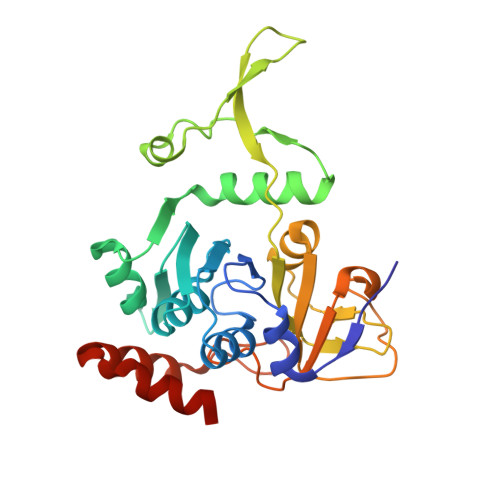
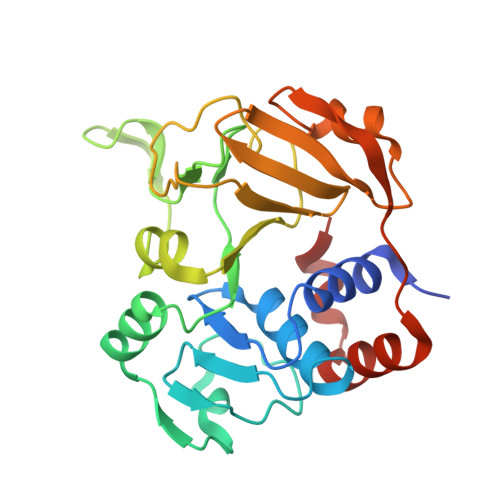
AibA/AibB Induces an Intramolecular Decarboxylation in Isovalerate Biosynthesis by Myxococcus xanthus.
Bock, T., Luxenburger, E., Hoffmann, J., Schutza, V., Feiler, C., Muller, R., Blankenfeldt, W.(2017) Angew Chem Int Ed Engl 56: 9986-9989
- PubMed: 28508504
- DOI: https://doi.org/10.1002/anie.201701992
- Primary Citation of Related Structures:
5MZW, 5MZX, 5MZY, 5MZZ, 5N00, 5N01, 5N02, 5N03 - PubMed Abstract:
Isovaleryl coenzyme A (IV-CoA) is an important precursor for iso-fatty acids and lipids. It acts in the development of myxobacteria, which can produce this compound from acetyl-CoA through alternative IV-CoA biosynthesis (aib). A central reaction of aib is catalyzed by AibA/AibB, which acts as a cofactor-free decarboxylase despite belonging to the family of CoA-transferases. We developed an efficient expression system for AibA/AibB that allowed the determination of high-resolution crystal structures in complex with different ligands. Through mutational studies, we show that an active-site cysteine previously proposed to be involved in decarboxylation is not required for activity. Instead, AibA/AibB seems to induce an intramolecular decarboxylation by binding its substrate in a hydrophobic cavity and forcing it into a bent conformation. Our study opens opportunities for synthetic biology studies, since AibA/AibB may be suitable for the production of isobutene, a precursor of biofuels and chemicals.
Organizational Affiliation:
Structure and Function of Proteins, Helmholtz Centre for Infection Research, I, nhoffenstr. 7, 38124, Braunschweig, Germany.