Chemically Induced Degradation of the Oncogenic Transcription Factor BCL6.
Kerres, N., Steurer, S., Schlager, S., Bader, G., Berger, H., Caligiuri, M., Dank, C., Engen, J.R., Ettmayer, P., Fischerauer, B., Flotzinger, G., Gerlach, D., Gerstberger, T., Gmaschitz, T., Greb, P., Han, B., Heyes, E., Iacob, R.E., Kessler, D., Kolle, H., Lamarre, L., Lancia, D.R., Lucas, S., Mayer, M., Mayr, K., Mischerikow, N., Muck, K., Peinsipp, C., Petermann, O., Reiser, U., Rudolph, D., Rumpel, K., Salomon, C., Scharn, D., Schnitzer, R., Schrenk, A., Schweifer, N., Thompson, D., Traxler, E., Varecka, R., Voss, T., Weiss-Puxbaum, A., Winkler, S., Zheng, X., Zoephel, A., Kraut, N., McConnell, D., Pearson, M., Koegl, M.(2017) Cell Rep 20: 2860-2875
- PubMed: 28930682
- DOI: https://doi.org/10.1016/j.celrep.2017.08.081
- Primary Citation of Related Structures:
5MW2, 5MW6, 5MWD - PubMed Abstract:
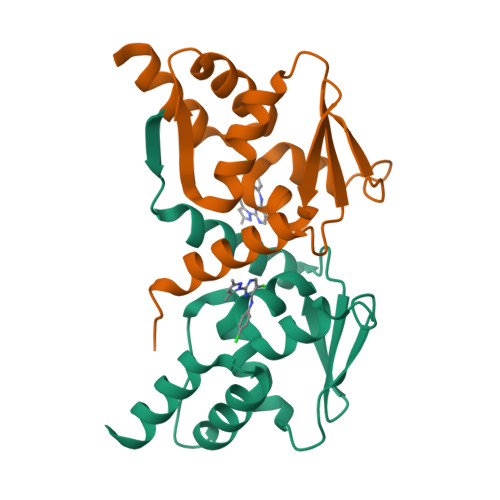
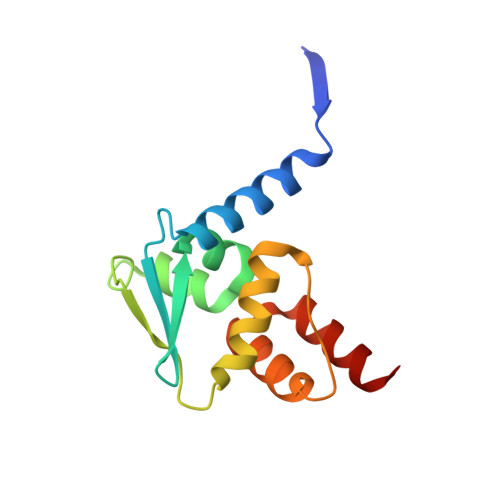
The transcription factor BCL6 is a known driver of oncogenesis in lymphoid malignancies, including diffuse large B cell lymphoma (DLBCL). Disruption of its interaction with transcriptional repressors interferes with the oncogenic effects of BCL6. We used a structure-based drug design to develop highly potent compounds that block this interaction. A subset of these inhibitors also causes rapid ubiquitylation and degradation of BCL6 in cells. These compounds display significantly stronger induction of expression of BCL6-repressed genes and anti-proliferative effects than compounds that merely inhibit co-repressor interactions. This work establishes the BTB domain as a highly druggable structure, paving the way for the use of other members of this protein family as drug targets. The magnitude of effects elicited by this class of BCL6-degrading compounds exceeds that of our equipotent non-degrading inhibitors, suggesting opportunities for the development of BCL6-based lymphoma therapeutics.
Organizational Affiliation:
Boehringer Ingelheim RCV GmbH & Co KG, 1221 Vienna, Austria.