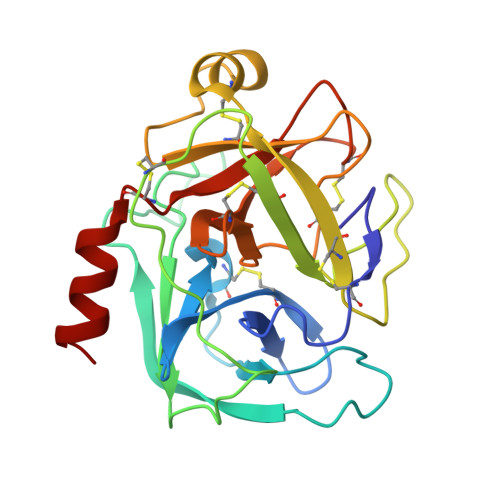
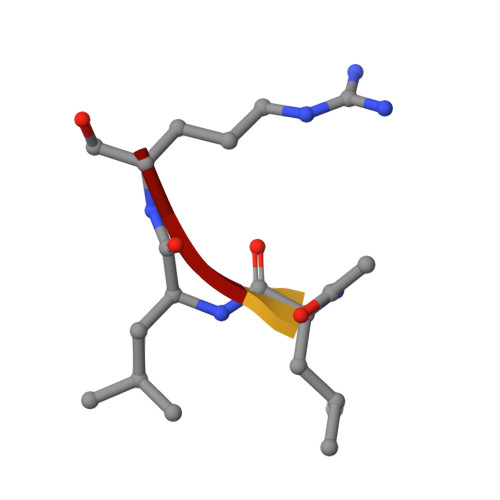
Structural determinants of specificity and regulation of activity in the allosteric loop network of human KLK8/neuropsin.
Debela, M., Magdolen, V., Skala, W., Elsasser, B., Schneider, E.L., Craik, C.S., Biniossek, M.L., Schilling, O., Bode, W., Brandstetter, H., Goettig, P.(2018) Sci Rep 8: 10705-10705
- PubMed: 30013126
- DOI: https://doi.org/10.1038/s41598-018-29058-6
- Primary Citation of Related Structures:
5MS4 - PubMed Abstract:
Human KLK8/neuropsin, a kallikrein-related serine peptidase, is mostly expressed in skin and the hippocampus regions of the brain, where it regulates memory formation by synaptic remodeling. Substrate profiles of recombinant KLK8 were analyzed with positional scanning using fluorogenic tetrapeptides and the proteomic PICS approach, which revealed the prime side specificity. Enzyme kinetics with optimized substrates showed stimulation by Ca 2+ and inhibition by Zn 2+ , which are physiological regulators. Crystal structures of KLK8 with a ligand-free active site and with the inhibitor leupeptin explain the subsite specificity and display Ca 2+ bound to the 75-loop. The variants D70K and H99A confirmed the antagonistic role of the cation binding sites. Molecular docking and dynamics calculations provided insights in substrate binding and the dual regulation of activity by Ca 2+ and Zn 2+ , which are important in neuron and skin physiology. Both cations participate in the allosteric surface loop network present in related serine proteases. A comparison of the positional scanning data with substrates from brain suggests an adaptive recognition by KLK8, based on the tertiary structures of its targets. These combined findings provide a comprehensive picture of the molecular mechanisms underlying the enzyme activity of KLK8.
Organizational Affiliation:
Max-Planck-Institut für Biochemie, Proteinase Research Group, 82152, Martinsried, Germany.