Structure and assembly of scalable porous protein cages.
Sasaki, E., Bohringer, D., van de Waterbeemd, M., Leibundgut, M., Zschoche, R., Heck, A.J., Ban, N., Hilvert, D.(2017) Nat Commun 8: 14663-14663
- PubMed: 28281548
- DOI: https://doi.org/10.1038/ncomms14663
- Primary Citation of Related Structures:
5MPP, 5MQ3, 5MQ7 - PubMed Abstract:
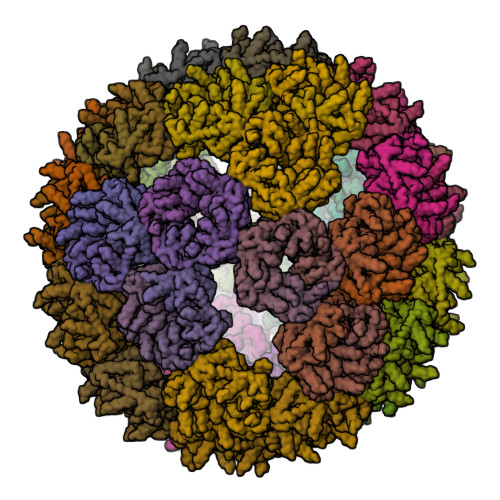
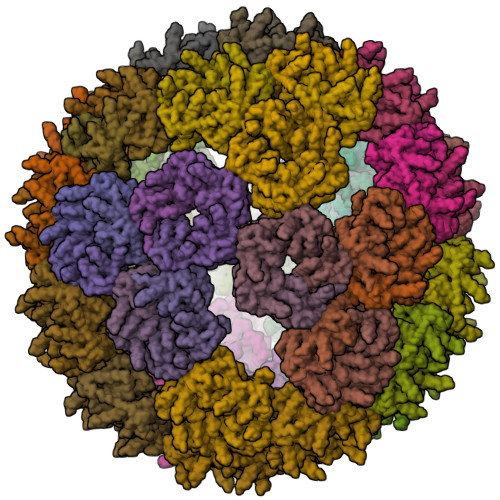
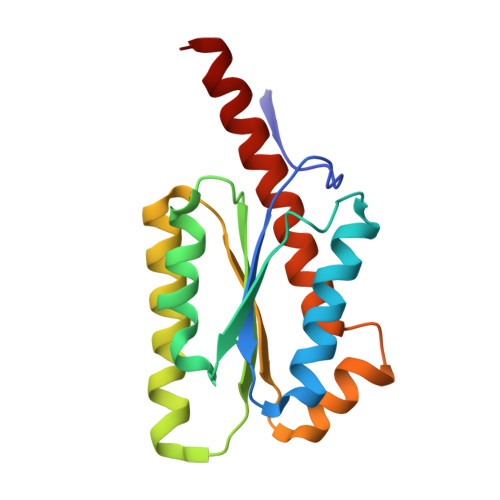
Proteins that self-assemble into regular shell-like polyhedra are useful, both in nature and in the laboratory, as molecular containers. Here we describe cryo-electron microscopy (EM) structures of two versatile encapsulation systems that exploit engineered electrostatic interactions for cargo loading. We show that increasing the number of negative charges on the lumenal surface of lumazine synthase, a protein that naturally assembles into a ∼1-MDa dodecahedron composed of 12 pentamers, induces stepwise expansion of the native protein shell, giving rise to thermostable ∼3-MDa and ∼6-MDa assemblies containing 180 and 360 subunits, respectively. Remarkably, these expanded particles assume unprecedented tetrahedrally and icosahedrally symmetric structures constructed entirely from pentameric units. Large keyhole-shaped pores in the shell, not present in the wild-type capsid, enable diffusion-limited encapsulation of complementarily charged guests. The structures of these supercharged assemblies demonstrate how programmed electrostatic effects can be effectively harnessed to tailor the architecture and properties of protein cages.
Organizational Affiliation:
Department of Chemistry and Applied Biosciences, Laboratory of Organic Chemistry, ETH Zürich, Zürich 8093, Switzerland.