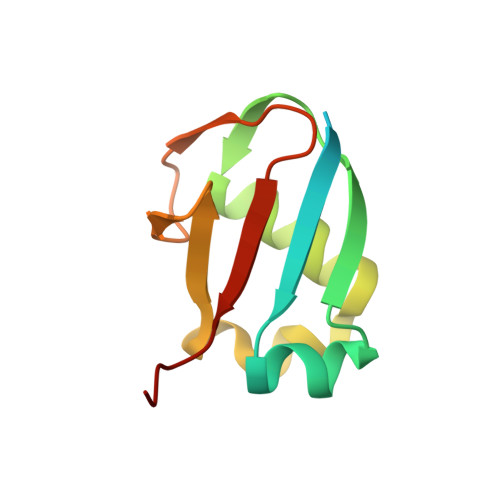
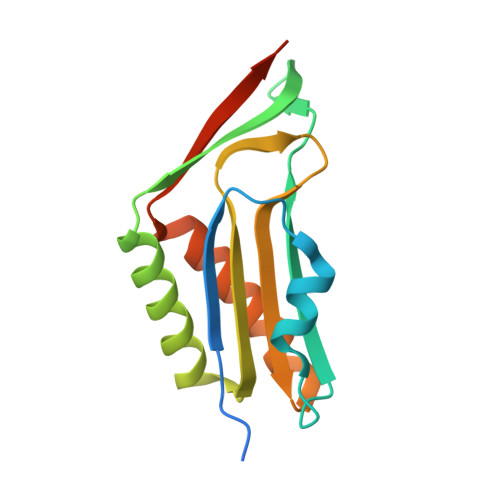
Crystal structure of human molybdopterin synthase complex
Kopec, J., Bailey, H., Fitzpatrick, F., Strain-Damerell, C., Oberholzer, A.E., Williams, E., Burgess-Brown, N., von Delft, F., Arrowsmith, C., Edwards, A., Bountra, C., Yue, W.W.To be published.