Discovery of BAZ2A bromodomain ligands.
Spiliotopoulos, D., Wamhoff, E.C., Lolli, G., Rademacher, C., Caflisch, A.(2017) Eur J Med Chem 139: 564-572
- PubMed: 28837921
- DOI: https://doi.org/10.1016/j.ejmech.2017.08.028
- Primary Citation of Related Structures:
5MGE, 5MGF, 5MGG, 5MGJ, 5MGK, 5MGL, 5MGM - PubMed Abstract:
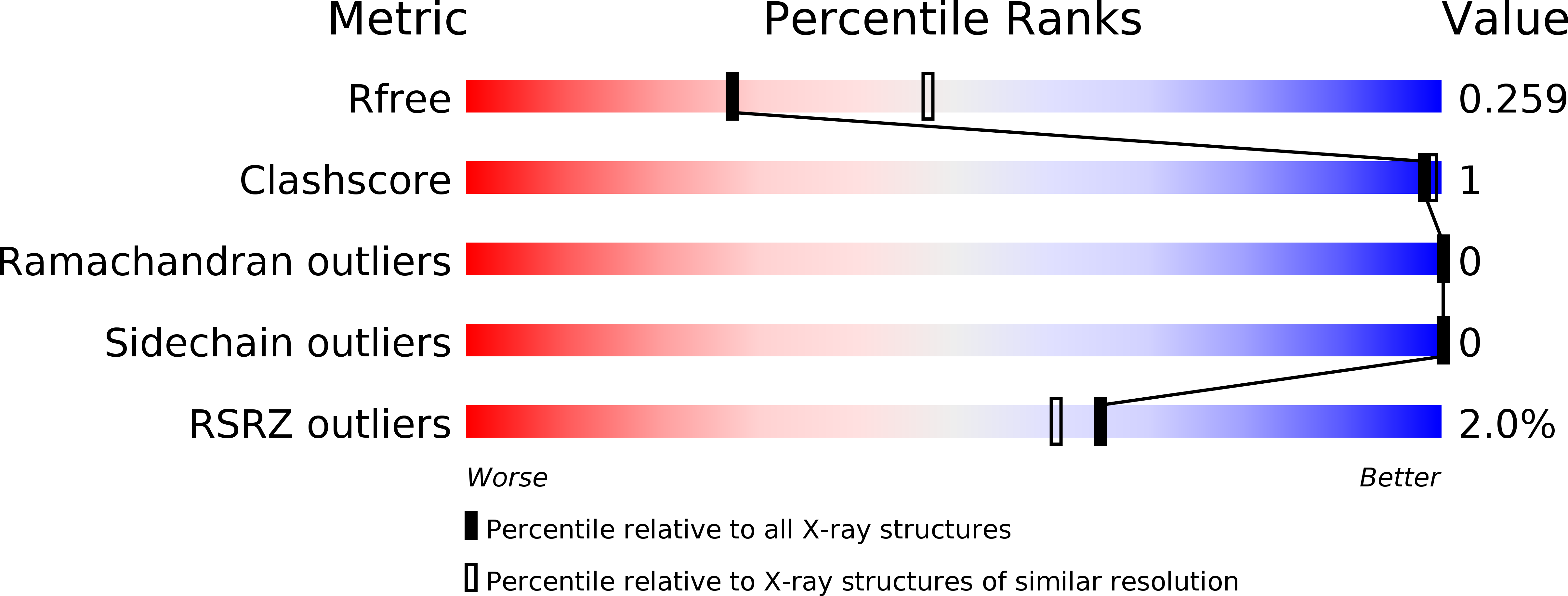
The bromodomain adjacent to zinc finger domain protein 2A (BAZ2A) is implicated in aggressive prostate cancer. The BAZ2A bromodomain is a challenging target because of the shallow pocket of its natural ligand, the acetylated side chain of lysine. Here, we report the successful screening of a library of nearly 1500 small molecules by high-throughput docking and force field-based binding-energy evaluation. For seven of the 20 molecules selected in silico, evidence of binding to the BAZ2A bromodomain is provided by ligand-observed NMR spectroscopy. Two of these compounds show a favorable ligand efficiency of 0.42 kcal/mol per non-hydrogen atom in a competition-binding assay. The crystal structures of the BAZ2A bromodomain in complex with four fragment hits validate the predicted binding modes. The binding modes of compounds 1 and 3 are compatible with ligand growing for optimization of affinity for BAZ2A and selectivity against the close homologue BAZ2B.
Organizational Affiliation:
Department of Biochemistry, University of Zürich, Winterthurerstrasse 190, CH-8057 Zürich, Switzerland.