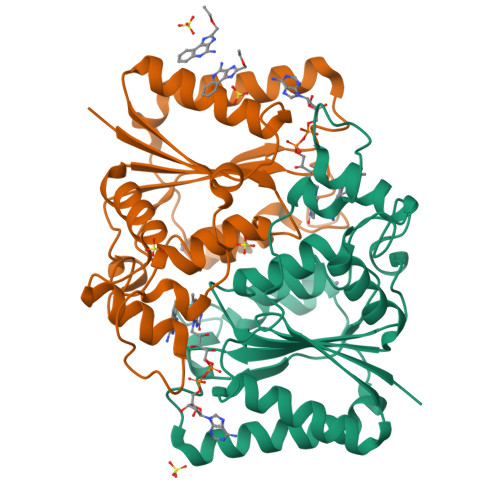
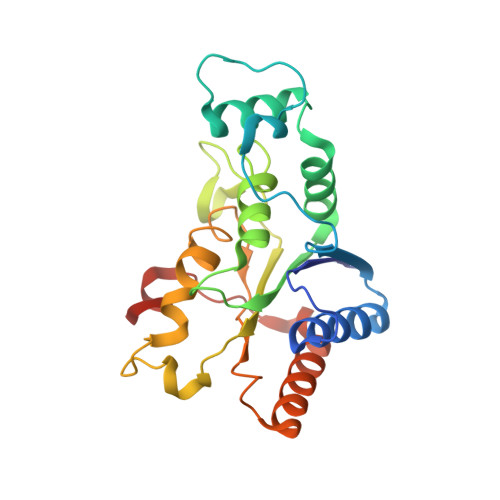
Imiquimod Inhibits Mitochondrial Complex I and Induces K+ efflux-independent Nlrp3 Inflammasome Activation via Nek7
Gross, C., Mishra, R., Schneider, K., Medard, G., Wettmarshausen, J., Dittlein, D., Gorka, O., Koenig, P.-A., Fromm, S., Magnani, G., Cikovic, T., Hartjes, L., Smollich, J., Robertson, A., Cooper, M., Schmidt-Supprian, M., Schuster, M., Schroder, K., Broz, P., Traidl-Hoffmann, C., Kuester, B., Ruland, J., Schneider, S., Perocchi, F., Gross, O.To be published.