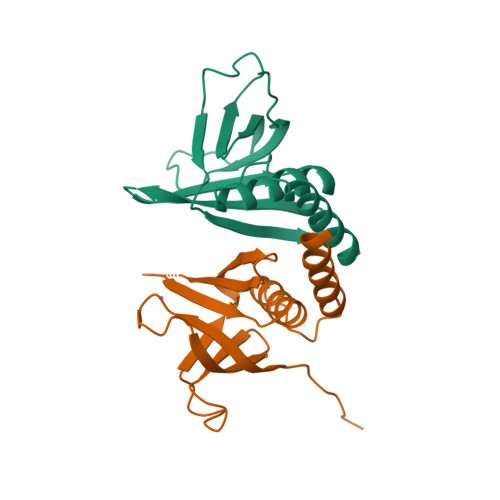
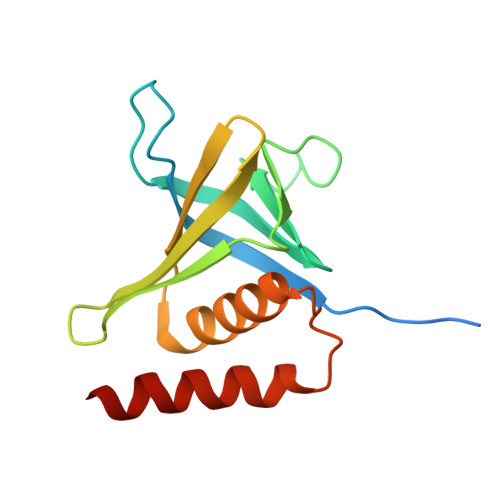
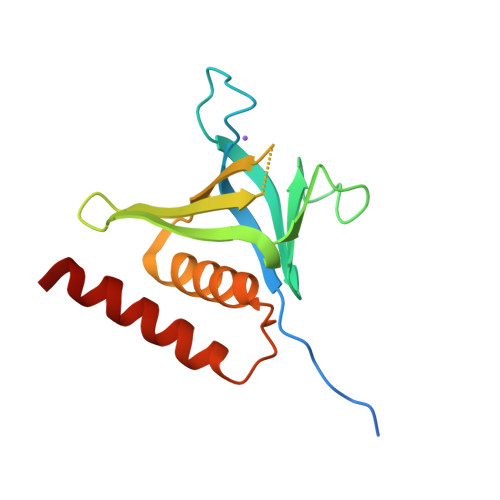
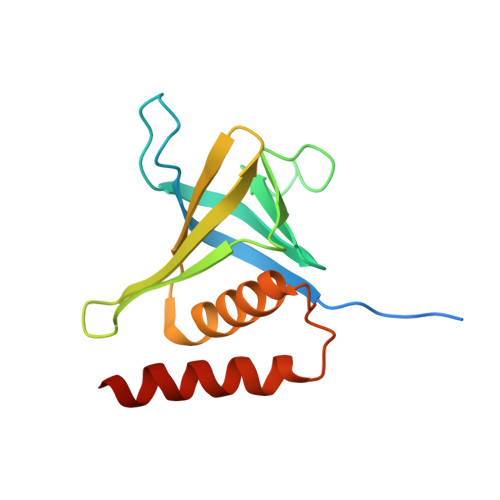
Structure and lipid-binding properties of the kindlin-3 pleckstrin homology domain.
Ni, T., Kalli, A.C., Naughton, F.B., Yates, L.A., Naneh, O., Kozorog, M., Anderluh, G., Sansom, M.S., Gilbert, R.J.(2017) Biochem J 474: 539-556
- PubMed: 27974389
- DOI: https://doi.org/10.1042/BCJ20160791
- Primary Citation of Related Structures:
5L81 - PubMed Abstract:
Kindlins co-activate integrins alongside talin. They possess, like talin, a FERM domain (4.1-erythrin-radixin-moiesin domain) comprising F0-F3 subdomains, but with a pleckstrin homology (PH) domain inserted in the F2 subdomain that enables membrane association. We present the crystal structure of murine kindlin-3 PH domain determined at a resolution of 2.23 Å and characterise its lipid binding using biophysical and computational approaches. Molecular dynamics simulations suggest flexibility in the PH domain loops connecting β-strands forming the putative phosphatidylinositol phosphate (PtdInsP)-binding site. Simulations with PtdInsP-containing bilayers reveal that the PH domain associates with PtdInsP molecules mainly via the positively charged surface presented by the β1-β2 loop and that it binds with somewhat higher affinity to PtdIns(3,4,5)P 3 compared with PtdIns(4,5)P 2 Surface plasmon resonance (SPR) with lipid headgroups immobilised and the PH domain as an analyte indicate affinities of 300 µM for PtdIns(3,4,5)P 3 and 1 mM for PtdIns(4,5)P 2 In contrast, SPR studies with an immobilised PH domain and lipid nanodiscs as the analyte show affinities of 0.40 µM for PtdIns(3,4,5)P 3 and no affinity for PtdIns(4,5)P 2 when the inositol phosphate constitutes 5% of the total lipids (∼5 molecules per nanodisc). Reducing the PtdIns(3,4,5)P 3 composition to 1% abolishes nanodisc binding to the PH domain, as does site-directed mutagenesis of two lysines within the β1-β2 loop. Binding of PtdIns(3,4,5)P 3 by a canonical PH domain, Grp1, is not similarly influenced by SPR experimental design. These data suggest a role for PtdIns(3,4,5)P 3 clustering in the binding of some PH domains and not others, highlighting the importance of lipid mobility and clustering for the biophysical assessment of protein-membrane interactions.
Organizational Affiliation:
Division of Structural Biology, Wellcome Trust Centre for Human Genetics, University of Oxford, Roosevelt Drive, Oxford OX3 7BN, U.K.