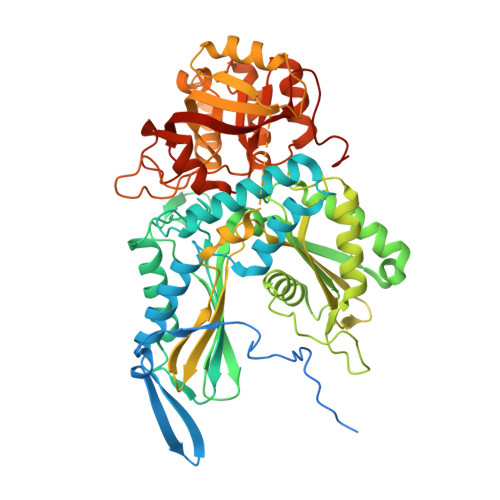
Crystal structure of an Fe-S cluster-containing fumarate hydratase enzyme from Leishmania major reveals a unique protein fold.
Feliciano, P.R., Drennan, C.L., Nonato, M.C.(2016) Proc Natl Acad Sci U S A 113: 9804-9809
- PubMed: 27528683
- DOI: https://doi.org/10.1073/pnas.1605031113
- Primary Citation of Related Structures:
5L2R - PubMed Abstract:
Fumarate hydratases (FHs) are essential metabolic enzymes grouped into two classes. Here, we present the crystal structure of a class I FH, the cytosolic FH from Leishmania major, which reveals a previously undiscovered protein fold that coordinates a catalytically essential [4Fe-4S] cluster. Our 2.05 Å resolution data further reveal a dimeric architecture for this FH that resembles a heart, with each lobe comprised of two domains that are arranged around the active site. Besides the active site, where the substrate S-malate is bound bidentate to the unique iron of the [4Fe-4S] cluster, other binding pockets are found near the dimeric enzyme interface, some of which are occupied by malonate, shown here to be a weak inhibitor of this enzyme. Taken together, these data provide a framework both for investigations of the class I FH catalytic mechanism and for drug design aimed at fighting neglected tropical diseases.
Organizational Affiliation:
Laboratório de Cristalografia de Proteínas, Faculdade de Ciências Farmacêuticas de Ribeirão Preto, Universidade de São Paulo, São Paulo 14040-903, Brazil; Department of Chemistry, Massachusetts Institute of Technology, Cambridge, MA 02139; Department of Biology, Massachusetts Institute of Technology, Cambridge, MA 02139;