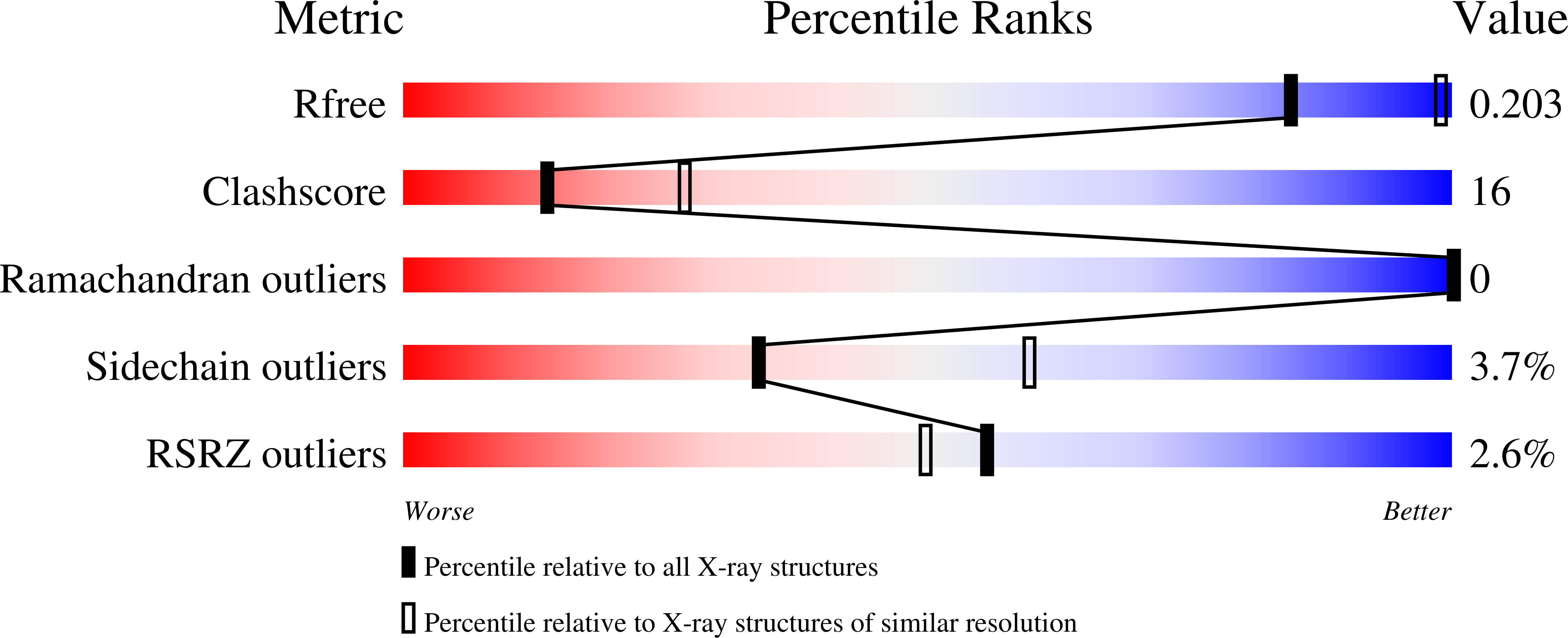
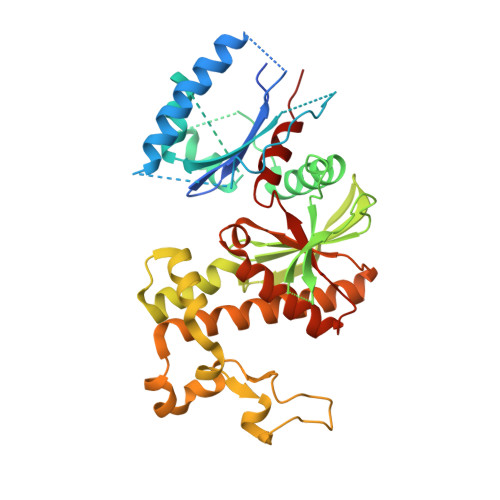
Allosteric Regulation of Mammalian Pantothenate Kinase.
Subramanian, C., Yun, M.K., Yao, J., Sharma, L.K., Lee, R.E., White, S.W., Jackowski, S., Rock, C.O.(2016) J Biol Chem 291: 22302-22314
- PubMed: 27555321
- DOI: https://doi.org/10.1074/jbc.M116.748061
- Primary Citation of Related Structures:
5KPR, 5KPT, 5KPZ, 5KQ8, 5KQD - PubMed Abstract:
Pantothenate kinase is the master regulator of CoA biosynthesis and is feedback-inhibited by acetyl-CoA. Comparison of the human PANK3·acetyl-CoA complex to the structures of PANK3 in four catalytically relevant complexes, 5'-adenylyl-β,γ-imidodiphosphate (AMPPNP)·Mg 2+ , AMPPNP·Mg 2+ ·pantothenate, ADP·Mg 2+ ·phosphopantothenate, and AMP phosphoramidate (AMPPN)·Mg 2+ , revealed a large conformational change in the dimeric enzyme. The amino-terminal nucleotide binding domain rotates to close the active site, and this allows the P-loop to engage ATP and facilitates required substrate/product interactions at the active site. Biochemical analyses showed that the transition between the inactive and active conformations, as assessed by the binding of either ATP·Mg 2+ or acyl-CoA to PANK3, is highly cooperative indicating that both protomers move in concert. PANK3(G19V) cannot bind ATP, and biochemical analyses of an engineered PANK3/PANK3(G19V) heterodimer confirmed that the two active sites are functionally coupled. The communication between the two protomers is mediated by an α-helix that interacts with the ATP-binding site at its amino terminus and with the substrate/inhibitor-binding site of the opposite protomer at its carboxyl terminus. The two α-helices within the dimer together with the bound ligands create a ring that stabilizes the assembly in either the active closed conformation or the inactive open conformation. Thus, both active sites of the dimeric mammalian pantothenate kinases coordinately switch between the on and off states in response to intracellular concentrations of ATP and its key negative regulators, acetyl(acyl)-CoA.
Organizational Affiliation:
From the Departments of Infectious Diseases.