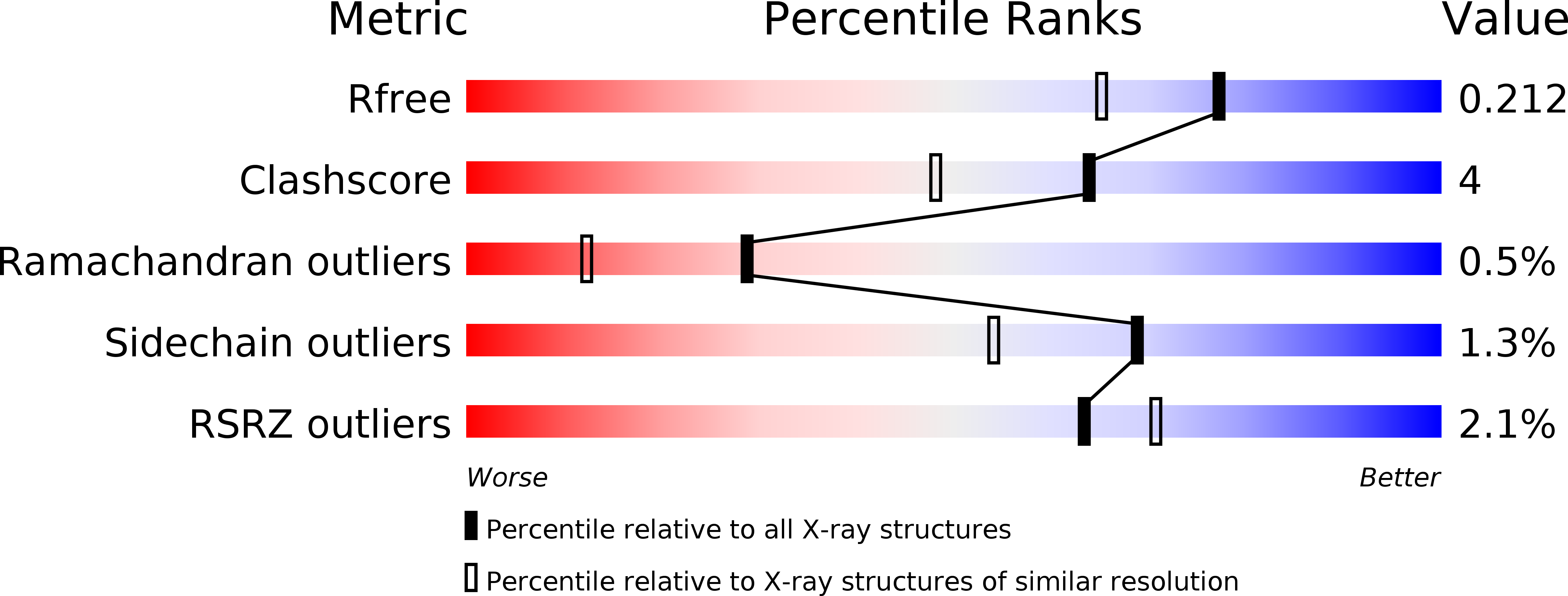
Dynamic Conformational States Dictate Selectivity toward the Native Substrate in a Substrate-Permissive Acyltransferase.
Levsh, O., Chiang, Y.C., Tung, C.F., Noel, J.P., Wang, Y., Weng, J.K.(2016) Biochemistry 55: 6314-6326
- PubMed: 27805809
- DOI: https://doi.org/10.1021/acs.biochem.6b00887
- Primary Citation of Related Structures:
5KJS, 5KJT, 5KJU, 5KJV, 5KJW - PubMed Abstract:
Hydroxycinnamoyl-CoA:shikimate hydroxycinnamoyltransferase (HCT) is an essential acyltransferase that mediates flux through plant phenylpropanoid metabolism by catalyzing a reaction between p-coumaroyl-CoA and shikimate, yet it also exhibits broad substrate permissiveness in vitro. How do enzymes like HCT avoid functional derailment by cellular metabolites that qualify as non-native substrates? Here, we combine X-ray crystallography and molecular dynamics to reveal distinct dynamic modes of HCT under native and non-native catalysis. We find that essential electrostatic and hydrogen-bonding interactions between the ligand and active site residues, permitted by active site plasticity, are elicited more effectively by shikimate than by other non-native substrates. This work provides a structural basis for how dynamic conformational states of HCT favor native over non-native catalysis by reducing the number of futile encounters between the enzyme and shikimate.
Organizational Affiliation:
Whitehead Institute for Biomedical Research , 9 Cambridge Center, Cambridge, Massachusetts 02142, United States.