NMR structure and dynamics of Q4DY78, a conserved kinetoplasid-specific protein from Trypanosoma cruzi.
D'Andrea, E.D., Retel, J.S., Diehl, A., Schmieder, P., Oschkinat, H., Pires, J.R.(2021) J Struct Biol 213: 107715-107715
- PubMed: 33705979
- DOI: https://doi.org/10.1016/j.jsb.2021.107715
- Primary Citation of Related Structures:
5KGQ - PubMed Abstract:
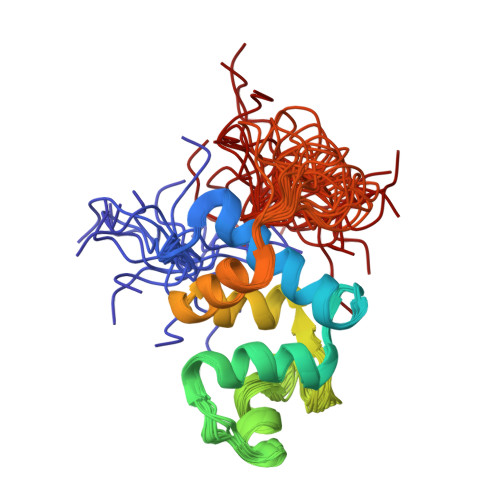
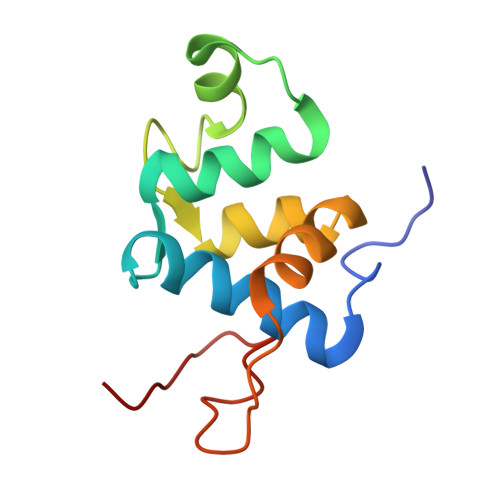
The 106-residue protein Q4DY78 (UniProt accession number) from Trypanosoma cruzi is highly conserved in the related kinetoplastid pathogens Trypanosoma brucei and Leishmania major. Given the essentiality of its orthologue in T. brucei, the high sequence conservation with other trypanosomatid proteins, and the low sequence similarity with mammalian proteins, Q4DY78 is an attractive protein for structural characterization. Here, we solved the structure of Q4DY78 by solution NMR and evaluated its backbone dynamics. Q4DY78 is composed of five α -helices and a small, two-stranded antiparallel β-sheet. The backbone RMSD is 0.22 ± 0.05 Å for the representative ensemble of the 20 lowest-energy structures. Q4DY78 is overall rigid, except for N-terminal residues (V 8 to I 10 ), residues at loop 4 (K 57 to G 65 ) and residues at the C-terminus (F 89 to F 112 ). Q4DY78 has a short motif FPCAP that could potentially mediate interactions with the host cytoskeleton via interaction with EVH1 (Drosophila Enabled (Ena)/Vasodilator-stimulated phosphoprotein (VASP) homology 1) domains. Albeit Q4DY78 lacks calcium-binding motifs, its fold resembles that of eukaryotic calcium-binding proteins such as calcitracin, calmodulin, and polcacin Bet V4. We characterized this novel protein with a calcium binding fold without the capacity to bind calcium.
Organizational Affiliation:
Instituto de Bioquímica Médica Leopoldo de Meis, Universidade Federal do Rio de Janeiro, Av. Carlos Chagas Filho, 373 - Bloco E, sala 32, Rio de Janeiro, RJ 21941-902, Brazil.