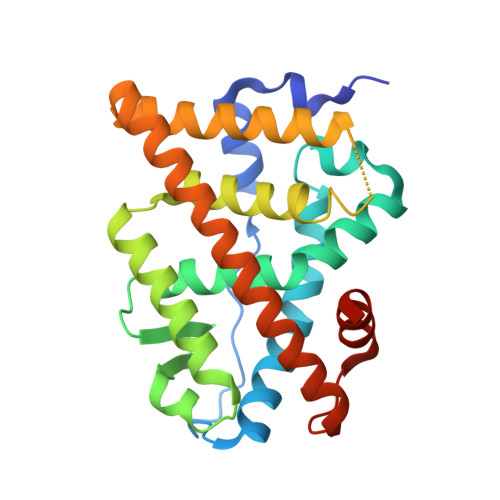
Full antagonism of the estrogen receptor without a prototypical ligand side chain.
Srinivasan, S., Nwachukwu, J.C., Bruno, N.E., Dharmarajan, V., Goswami, D., Kastrati, I., Novick, S., Nowak, J., Cavett, V., Zhou, H.B., Boonmuen, N., Zhao, Y., Min, J., Frasor, J., Katzenellenbogen, B.S., Griffin, P.R., Katzenellenbogen, J.A., Nettles, K.W.(2017) Nat Chem Biol 13: 111-118
- PubMed: 27870835
- DOI: https://doi.org/10.1038/nchembio.2236
- Primary Citation of Related Structures:
5KCC, 5KCD, 5KCE, 5KCF, 5KCT, 5KCU, 5KCW, 5KD9 - PubMed Abstract:
Resistance to endocrine therapies remains a major clinical problem for the treatment of estrogen receptor-α (ERα)-positive breast cancer. On-target side effects limit therapeutic compliance and use for chemoprevention, highlighting an unmet need for new therapies. Here we present a full-antagonist ligand series lacking the prototypical ligand side chain that has been universally used to engender antagonism of ERα through poorly understood structural mechanisms. A series of crystal structures and phenotypic assays reveal a structure-based design strategy with separate design elements for antagonism and degradation of the receptor, and access to a structurally distinct space for further improvements in ligand design. Understanding structural rules that guide ligands to produce diverse ERα-mediated phenotypes has broad implications for the treatment of breast cancer and other estrogen-sensitive aspects of human health including bone homeostasis, energy metabolism, and autoimmunity.
Organizational Affiliation:
Department of Cancer Biology, The Scripps Research Institute, Jupiter, Florida, USA.