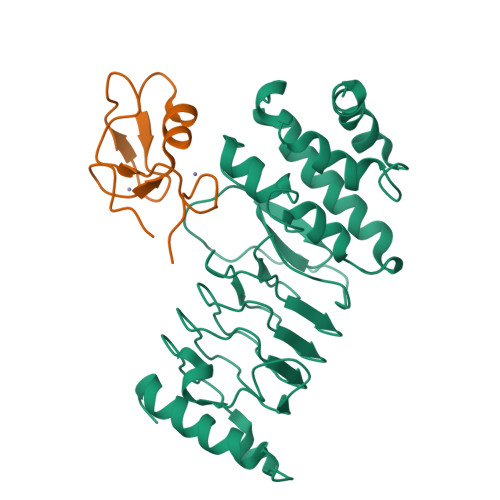
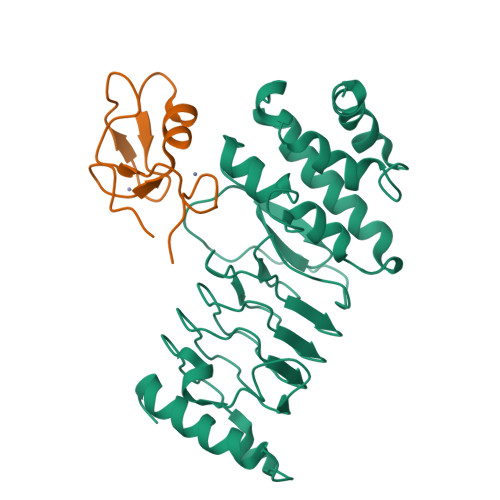
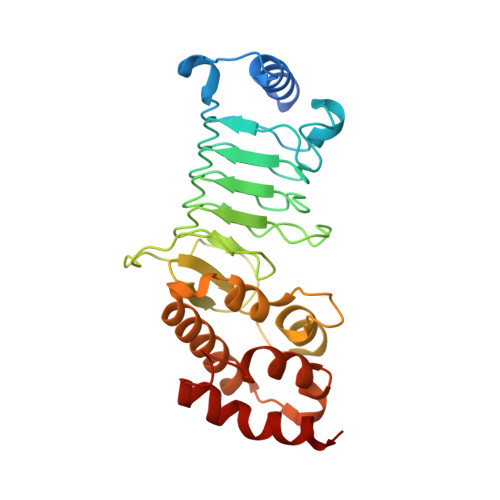
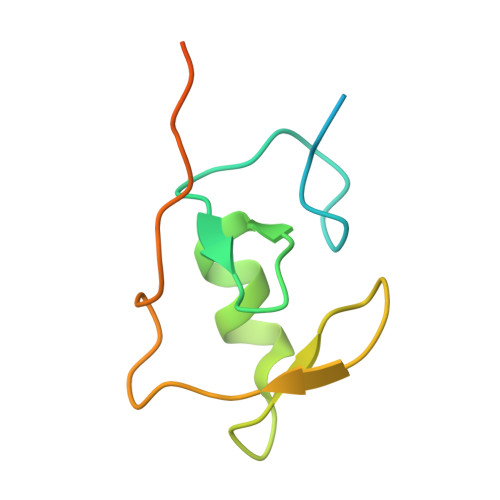
Structural basis for the recognition and degradation of host TRIM proteins by Salmonella effector SopA.
Fiskin, E., Bhogaraju, S., Herhaus, L., Kalayil, S., Hahn, M., Dikic, I.(2017) Nat Commun 8: 14004-14004
- PubMed: 28084320
- DOI: https://doi.org/10.1038/ncomms14004
- Primary Citation of Related Structures:
5JW7 - PubMed Abstract:
The hallmark of Salmonella Typhimurium infection is an acute intestinal inflammatory response, which is mediated through the action of secreted bacterial effector proteins. The pro-inflammatory Salmonella effector SopA is a HECT-like E3 ligase, which was previously proposed to activate host RING ligases TRIM56 and TRIM65. Here we elucidate an inhibitory mechanism of TRIM56 and TRIM65 targeting by SopA. We present the crystal structure of SopA in complex with the RING domain of human TRIM56, revealing the atomic details of their interaction and the basis for SopA selectivity towards TRIM56 and TRIM65. Structure-guided biochemical analysis shows that SopA inhibits TRIM56 E3 ligase activity by occluding the E2-interacting surface of TRIM56. We further demonstrate that SopA ubiquitinates TRIM56 and TRIM65, resulting in their proteasomal degradation during infection. Our results provide the basis for how a bacterial HECT ligase blocks host RING ligases and exemplifies the multivalent power of bacterial effectors during infection.
Organizational Affiliation:
Institute of Biochemistry II, Goethe University School of Medicine, Theodor-Stern-Kai 7, 60590 Frankfurt am Main, Germany.