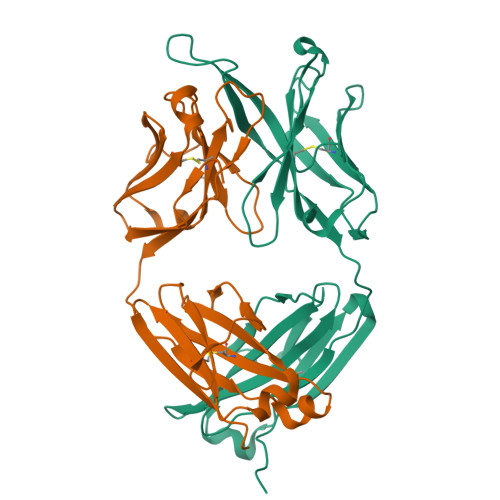
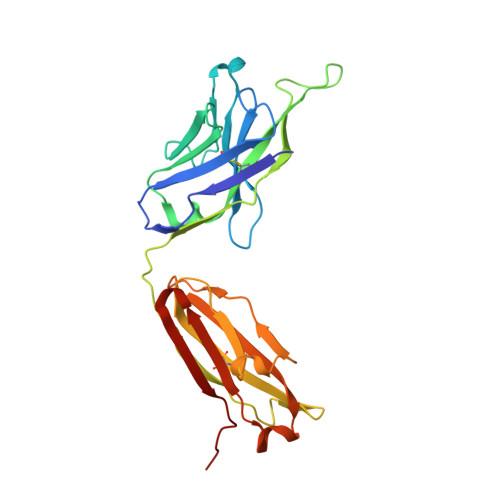
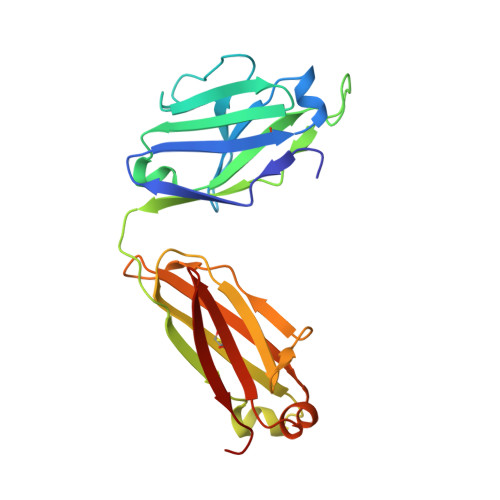
Developmental Pathway of the MPER-Directed HIV-1-Neutralizing Antibody 10E8.
Soto, C., Ofek, G., Joyce, M.G., Zhang, B., McKee, K., Longo, N.S., Yang, Y., Huang, J., Parks, R., Eudailey, J., Lloyd, K.E., Alam, S.M., Haynes, B.F., Mullikin, J.C., Connors, M., Mascola, J.R., Shapiro, L., Kwong, P.D.(2016) PLoS One 11: e0157409-e0157409
- PubMed: 27299673
- DOI: https://doi.org/10.1371/journal.pone.0157409
- Primary Citation of Related Structures:
5JNY, 5JO5, 5JR1 - PubMed Abstract:
Antibody 10E8 targets the membrane-proximal external region (MPER) of HIV-1 gp41, neutralizes >97% of HIV-1 isolates, and lacks the auto-reactivity often associated with MPER-directed antibodies. The developmental pathway of 10E8 might therefore serve as a promising template for vaccine design, but samples from time-of-infection-often used to infer the B cell record-are unavailable. In this study, we used crystallography, next-generation sequencing (NGS), and functional assessments to infer the 10E8 developmental pathway from a single time point. Mutational analysis indicated somatic hypermutation of the 2nd-heavy chain-complementarity determining region (CDR H2) to be critical for neutralization, and structures of 10E8 variants with V-gene regions reverted to genomic origin for heavy-and-light chains or heavy chain-only showed structural differences >2 Å relative to mature 10E8 in the CDR H2 and H3. To understand these developmental changes, we used bioinformatic sieving, maximum likelihood, and parsimony analyses of immunoglobulin transcripts to identify 10E8-lineage members, to infer the 10E8-unmutated common ancestor (UCA), and to calculate 10E8-developmental intermediates. We were assisted in this analysis by the preservation of a critical D-gene segment, which was unmutated in most 10E8-lineage sequences. UCA and early intermediates weakly bound a 26-residue-MPER peptide, whereas HIV-1 neutralization and epitope recognition in liposomes were only observed with late intermediates. Antibody 10E8 thus develops from a UCA with weak MPER affinity and substantial differences in CDR H2 and H3 from the mature 10E8; only after extensive somatic hypermutation do 10E8-lineage members gain recognition in the context of membrane and HIV-1 neutralization.
Organizational Affiliation:
Vaccine Research Center, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, Maryland, United States of America.