Crystal structure of iron uptake ABC transporter substrate-binding protein PiaA from Streptococcus pneumoniae Canada MDR_19A bound to hydroxymate siderophore ferrioxamine E and iron(III)
Stogios, P.J.To be published.
Experimental Data Snapshot
Starting Model: experimental
View more details
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| ABC transporter substrate-binding protein-iron transport | 308 | Streptococcus pneumoniae | Mutation(s): 0 Gene Names: yhfQ, yhfQ_1, yhfQ_2, yhfQ_3 | 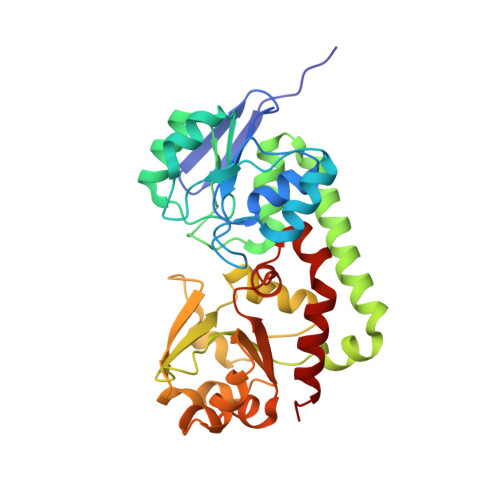 | |
UniProt | |||||
Find proteins for A0A062WLD9 (Streptococcus pneumoniae) Explore A0A062WLD9 Go to UniProtKB: A0A062WLD9 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | A0A062WLD9 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Ligands 4 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| 6L0 Query on 6L0 | D [auth A], I [auth B] | (8E)-6,17,28-trihydroxy-1,6,12,17,23,28-hexaazacyclotritriacont-8-ene-2,5,13,16,24,27-hexone C27 H46 N6 O9 SHTNNSLDUIEFSM-LREOWRDNSA-N |  | ||
| GOL Query on GOL | F [auth A], G [auth A], J [auth B], K [auth B] | GLYCEROL C3 H8 O3 PEDCQBHIVMGVHV-UHFFFAOYSA-N |  | ||
| FE Query on FE | C [auth A], H [auth B] | FE (III) ION Fe VTLYFUHAOXGGBS-UHFFFAOYSA-N |  | ||
| CL Query on CL | E [auth A] | CHLORIDE ION Cl VEXZGXHMUGYJMC-UHFFFAOYSA-M |  | ||
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 77.809 | α = 90 |
| b = 73.844 | β = 115.79 |
| c = 87.161 | γ = 90 |
| Software Name | Purpose |
|---|---|
| PHENIX | refinement |
| HKL-3000 | data reduction |
| HKL-3000 | data scaling |
| PHENIX | phasing |
| PHENIX | model building |
| Coot | model building |