Structure-Based Design of beta 5c Selective Inhibitors of Human Constitutive Proteasomes.
Xin, B.T., de Bruin, G., Huber, E.M., Besse, A., Florea, B.I., Filippov, D.V., van der Marel, G.A., Kisselev, A.F., van der Stelt, M., Driessen, C., Groll, M., Overkleeft, H.S.(2016) J Med Chem 59: 7177-7187
- PubMed: 27438186
- DOI: https://doi.org/10.1021/acs.jmedchem.6b00705
- Primary Citation of Related Structures:
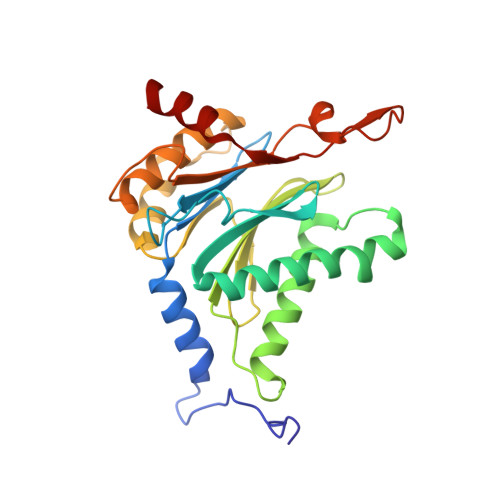
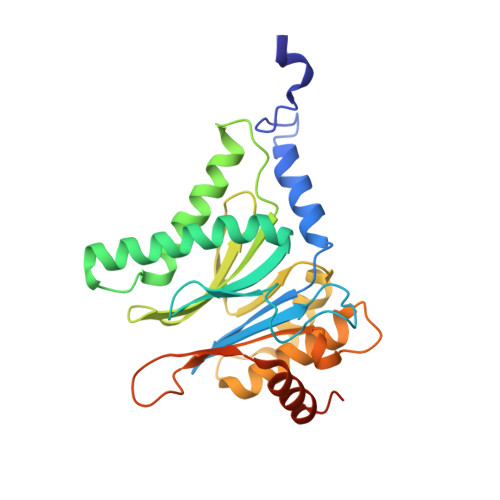
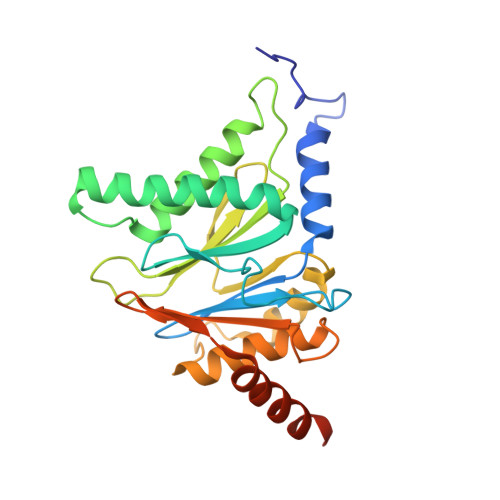
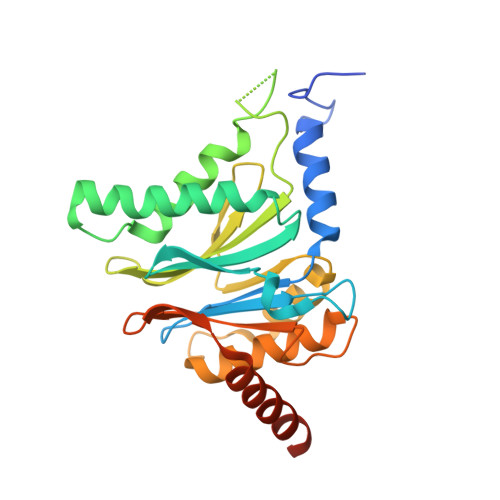
5JHR, 5JHS - PubMed Abstract:
This work reports the development of highly potent and selective inhibitors of the β5c catalytic activity of human constitutive proteasomes. The work describes the design principles, large hydrophobic P3 residue and small hydrophobic P1 residue, that led to the synthesis of a panel of peptide epoxyketones; their evaluation and the selection of the most promising compounds for further analyses. Structure-activity relationships detail how in a logical order the β1c/i, β2c/i, and β5i activities became resistant to inhibition as compounds were diversified stepwise. The most effective compounds were obtained as a mixture of cis- and trans-biscyclohexyl isomers, and enantioselective synthesis resolved this issue. Studies on yeast proteasome structures complexed with some of the compounds provide a rationale for the potency and specificity. Substitution of the N-terminus in the most potent compound for a more soluble equivalent led to a cell-permeable molecule that selectively and efficiently blocks β5c in cells expressing both constitutive proteasomes and immunoproteasomes.
Organizational Affiliation:
Gorlaeus Laboratories, Leiden Institute of Chemistry , Einsteinweg 55, 2333 CC Leiden, The Netherlands.