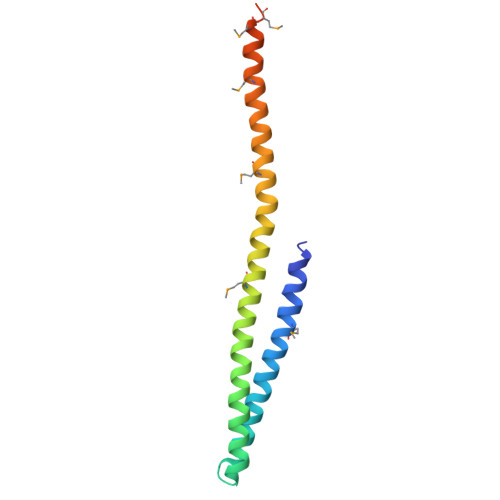
Electrostatic Interactions between Elongated Monomers Drive Filamentation of Drosophila Shrub, a Metazoan ESCRT-III Protein.
McMillan, B.J., Tibbe, C., Jeon, H., Drabek, A.A., Klein, T., Blacklow, S.C.(2016) Cell Rep 16: 1211-1217
- PubMed: 27452459
- DOI: https://doi.org/10.1016/j.celrep.2016.06.093
- Primary Citation of Related Structures:
5J45 - PubMed Abstract:
The endosomal sorting complex required for transport (ESCRT) is a conserved protein complex that facilitates budding and fission of membranes. It executes a key step in many cellular events, including cytokinesis and multi-vesicular body formation. The ESCRT-III protein Shrub in flies, or its homologs in yeast (Snf7) or humans (CHMP4B), is a critical polymerizing component of ESCRT-III needed to effect membrane fission. We report the structural basis for polymerization of Shrub and define a minimal region required for filament formation. The X-ray structure of the Shrub core shows that individual monomers in the lattice interact in a staggered arrangement using complementary electrostatic surfaces. Mutations that disrupt interface salt bridges interfere with Shrub polymerization and function. Despite substantial sequence divergence and differences in packing interactions, the arrangement of Shrub subunits in the polymer resembles that of Snf7 and other family homologs, suggesting that this intermolecular packing mechanism is shared among ESCRT-III proteins.
Organizational Affiliation:
Department of Biological Chemistry and Molecular Pharmacology, Harvard Medical School, Boston, MA 02115, USA.