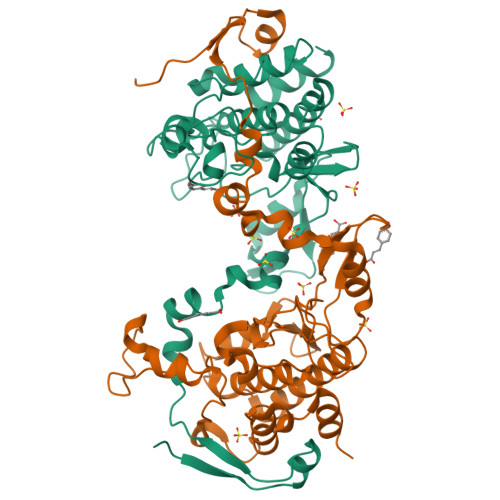
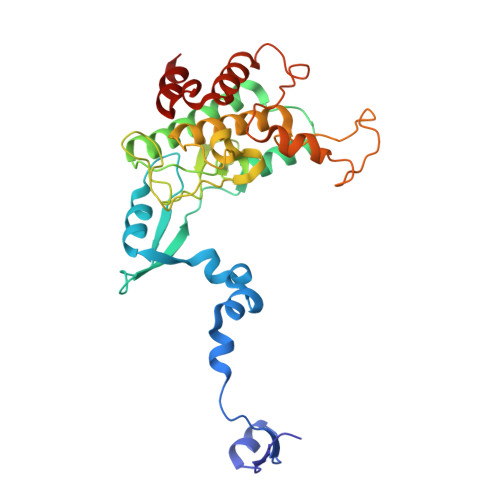
The crystal structure of an inactive dimer of PDZ-binding kinase
Dong, C., Tang, X., Xie, Y., Zou, Q., Yang, X., Zhou, H.(2016) Biochem Biophys Res Commun 476: 586-593
- PubMed: 27262437
- DOI: https://doi.org/10.1016/j.bbrc.2016.05.166
- Primary Citation of Related Structures:
5J0A - PubMed Abstract:
The overexpression of PDZ-binding kinase/T-LAK cell-originated protein kinase (PBK/TOPK) has been associated with hematologic tumors, breast cancer and various other cancers. However, the three-dimensional structure of PBK has not been solved. In this study, we determined the crystal structure of human PBK, which has two phospho-mimicking mutations T9E and T198E. The structural data indicated that PBK may assemble into an inactive dimer in alkaline conditions. Analytical size-exclusion chromatography and analytical ultracentrifugation confirmed that PBK exists in a conformational transition between dimers and monomers at different pH conditions. Co-IP and kinase assays suggested that the active state of PBK is a monomer and does not form a dimer even under alkaline conditions. These results showed that the conformational transition of PBK is important for its kinase activity regulation. Collectively, our observations may provide a novel starting point for structure-based functional studies.
Organizational Affiliation:
College of Life Sciences, Nankai University, 94 Weijin Road, Tianjin 300071, China.