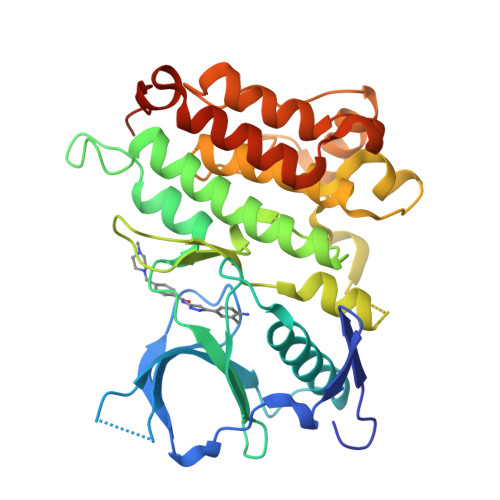
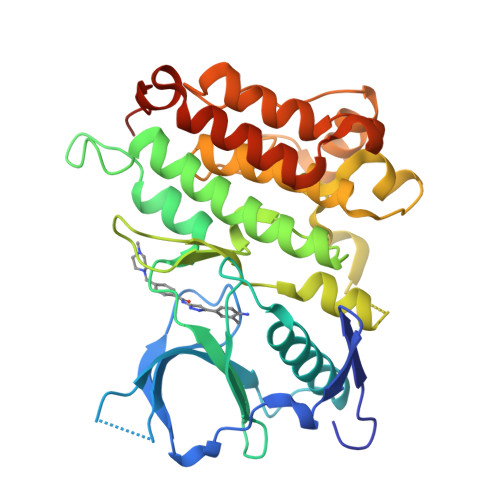
Pyrazolylamine Derivatives Reveal the Conformational Switching between Type I and Type II Binding Modes of Anaplastic Lymphoma Kinase (ALK).
Tu, C.H., Lin, W.H., Peng, Y.H., Hsu, T., Wu, J.S., Chang, C.Y., Lu, C.T., Lyu, P.C., Shih, C., Jiaang, W.T., Wu, S.Y.(2016) J Med Chem 59: 3906-3919
- PubMed: 27031565
- DOI: https://doi.org/10.1021/acs.jmedchem.6b00106
- Primary Citation of Related Structures:
5IUG, 5IUH, 5IUI - PubMed Abstract:
Most anaplastic lymphoma kinase (ALK) inhibitors adopt a type I binding mode, but only limited type II ALK structural studies are available. Herein, we present the structure of ALK in complex with N1-(3-4-[([5-(tert-butyl)-3-isoxazolyl]aminocarbonyl)amino]-3-methylphenyl-1H-5-pyrazolyl)-4-[(4-methylpiperazino)methyl]benzamide (5a), a novel ALK inhibitor adopting a type II binding mode. It revealed binding of 5a resulted in the conformational change and reposition of the activation loop, αC-helix, and juxtamembrane domain, which are all important domains for the autoinhibition mechanism and downstream signal pathway regulation of ALK. A structure-activity relationship study revealed that modifications to the structure of 5a led to significant differences in the ALK potency and altered the protein structure of ALK. To the best of our knowledge, this is the first structural biology study to directly observe how changes in the structure of a small molecule can regulate the switch between the type I and type II binding modes and induce dramatic conformational changes.
Organizational Affiliation:
Institute of Biotechnology and Pharmaceutical Research, National Health Research Institutes, 35 Keyan Road, Zhunan Town, Miaoli County 350, Taiwan, ROC.