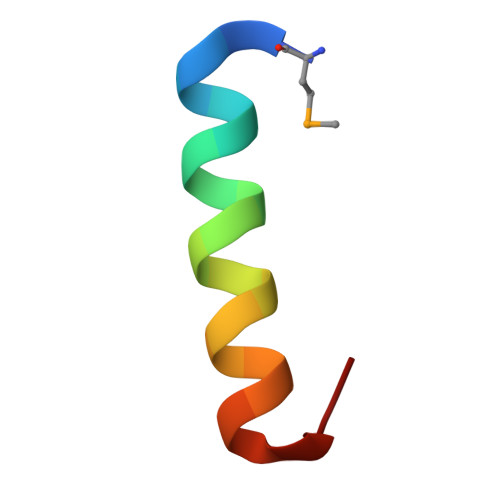
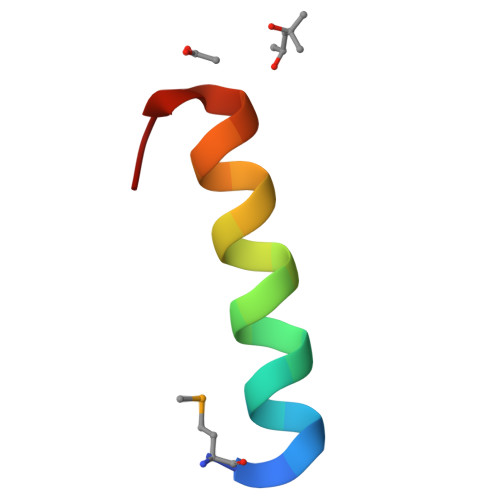The cytotoxic Staphylococcus aureus PSM alpha 3 reveals a cross-alpha amyloid-like fibril.
Tayeb-Fligelman, E., Tabachnikov, O., Moshe, A., Goldshmidt-Tran, O., Sawaya, M.R., Coquelle, N., Colletier, J.P., Landau, M.(2017) Science 355: 831-833
- PubMed: 28232575
- DOI: https://doi.org/10.1126/science.aaf4901
- Primary Citation of Related Structures:
5I55 - PubMed Abstract:
Amyloids are ordered protein aggregates, found in all kingdoms of life, and are involved in aggregation diseases as well as in physiological activities. In microbes, functional amyloids are often key virulence determinants, yet the structural basis for their activity remains elusive. We determined the fibril structure and function of the highly toxic, 22-residue phenol-soluble modulin α3 (PSMα3) peptide secreted by Staphylococcus aureus PSMα3 formed elongated fibrils that shared the morphological and tinctorial characteristics of canonical cross-β eukaryotic amyloids. However, the crystal structure of full-length PSMα3, solved de novo at 1.45 angstrom resolution, revealed a distinctive "cross-α" amyloid-like architecture, in which amphipathic α helices stacked perpendicular to the fibril axis into tight self-associating sheets. The cross-α fibrillation of PSMα3 facilitated cytotoxicity, suggesting that this assembly mode underlies function in S. aureus .
Organizational Affiliation:
Department of Biology, Technion-Israel Institute of Technology, Haifa 3200003, Israel.