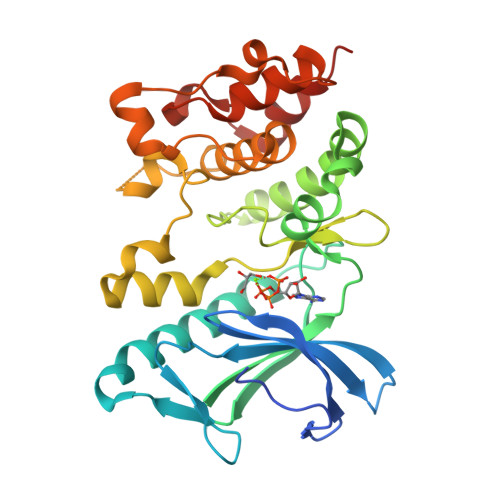
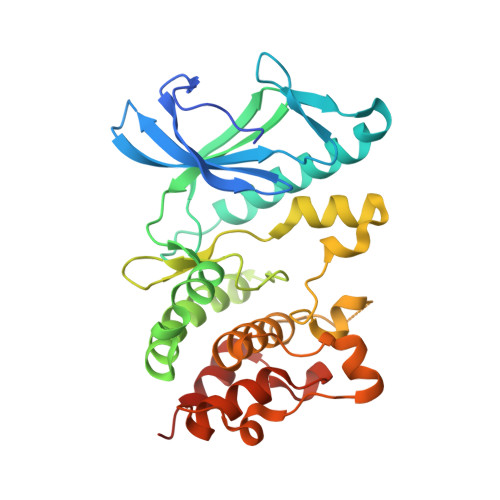
Crystal structures of the kinase domain of PpkA, a key regulatory component of T6SS, reveal a general inhibitory mechanism.
Li, P.P., Xu, D.Q., Ma, T.Q., Wang, D.Y., Li, W.D., He, J.H., Ran, T.T., Wang, W.W.(2018) Biochem J 475: 2209-2224
- PubMed: 29858276
- DOI: https://doi.org/10.1042/BCJ20180077
- Primary Citation of Related Structures:
5HNV, 5X1Q, 5X1R, 5X1S, 5X1T - PubMed Abstract:
The type VI secretion system (T6SS) is a versatile and widespread export system found in many Gram-negative bacteria that delivers effector proteins into target cells. The functions of T6SSs are tightly regulated by diverse mechanisms at multiple levels, including post-translational modification through threonine phosphorylation via the Ser/Thr protein kinase (STPK) PpkA. Here, we identified that PpkA is essential for T6SS secretion in Serratia marcescens since its deletion eliminated the secretion of haemolysin co-regulated protein, while the periplasmic and transmembrane portion of PpkA was found to be disposable for T6SS secretion. We further determined the crystal structure of the kinase domain of PpkA (PpkA-294). The structure of PpkA-294 was determined in its apo form to a 1.6 Å resolution as well as in complex with ATP to a 1.41 Å resolution and with an ATP analogue AMP-PCP to a 1.45 Å resolution. The residues in the activation loop of PpkA-294 were fully determined, and the N-terminus of the loop was folded into an unprecedented inhibitory helix, revealing that the PpkA kinase domain was in an auto-inhibitory state. The ternary MgATP-PpkA-294 complex was also inactive with nucleotide ribose and phosphates in unexpected and unproductive conformations. The αC-helix in the inactive PpkA-294 adopted a conformation towards the active site but with the conserved glutamate in the helix rotated away, which we suggest to be a general conformation for all STPK kinases in the inactive form. Structural comparison of PpkA with its eukaryotic homologues reinforced the universal regulation mechanism of protein kinases.
Organizational Affiliation:
Department of Microbiology, College of Life Sciences, Nanjing Agricultural University, 210095 Nanjing, PR China.