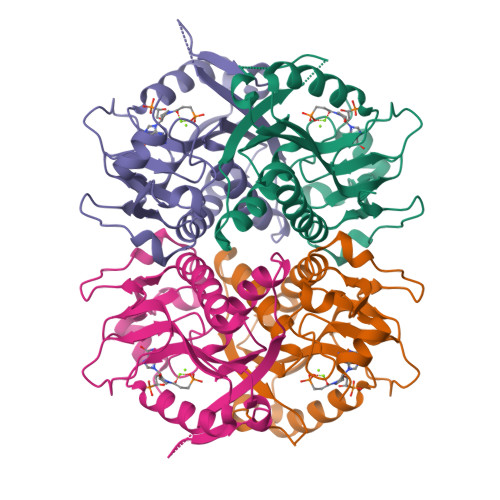
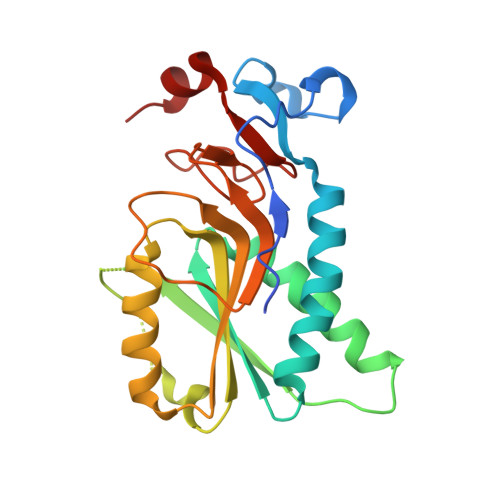
Design of Plasmodium vivax Hypoxanthine-Guanine Phosphoribosyltransferase Inhibitors as Potential Antimalarial Therapeutics.
Keough, D.T., Rejman, D., Pohl, R., Zbornikova, E., Hockova, D., Croll, T., Edstein, M.D., Birrell, G.W., Chavchich, M., Naesens, L.M.J., Pierens, G.K., Brereton, I.M., Guddat, L.W.(2017) ACS Chem Biol
- PubMed: 29161011
- DOI: https://doi.org/10.1021/acschembio.7b00916
- Primary Citation of Related Structures:
5HIA, 6BNJ, 6BO7 - PubMed Abstract:
Plasmodium falciparum (Pf) and Plasmodium vivax (Pv) are the foremost causative agents of malaria. Due to the development of resistance to current antimalarial medications, new drugs for this parasitic disease need to be discovered. The activity of hypoxanthine-guanine-[xanthine]-phosphoribosyltransferase, HG[X]PRT, is reported to be essential for the growth of both of these parasites, making it an excellent target for antimalarial drug discovery. Here, we have used rational structure-based methods to design an inhibitor, [3R,4R]-4-guanin-9-yl-3-((S)-2-hydroxy-2-phosphonoethyl)oxy-1-N-(phosphonopropionyl)pyrrolidine, of PvHGPRT and PfHGXPRT that has K i values of 8 and 7 nM, respectively, for these two enzymes. The crystal structure of PvHGPRT in complex with this compound has been determined to 2.85 Å resolution. The corresponding complex with human HGPRT was also obtained to allow a direct comparison of the binding modes of this compound with the two enzymes. The tetra-(ethyl l-phenylalanine) tetraamide prodrug of this compound was synthesized, and it has an IC 50 of 11.7 ± 3.2 μM against Pf lines grown in culture and a CC 50 in human A549 cell lines of 102 ± 11 μM, thus giving it a ∼10-fold selectivity index.
Organizational Affiliation:
School of Chemistry and Molecular Biosciences, The University of Queensland , Brisbane 4072, Australia.