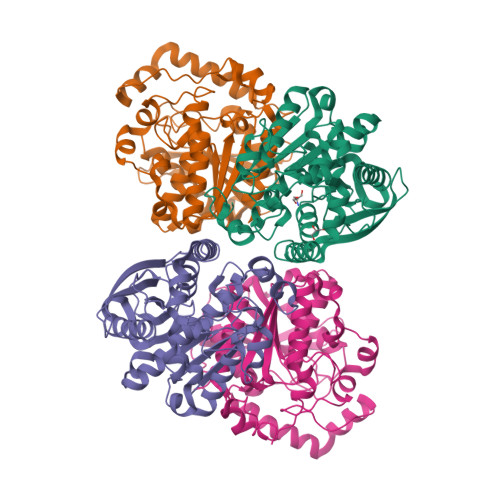
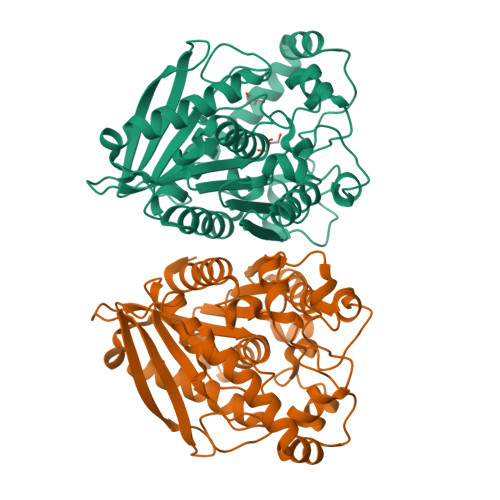
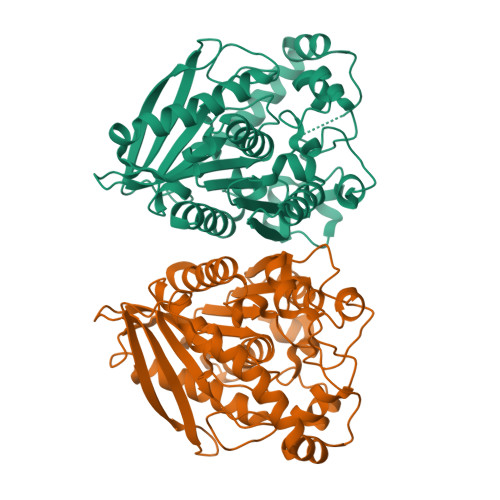
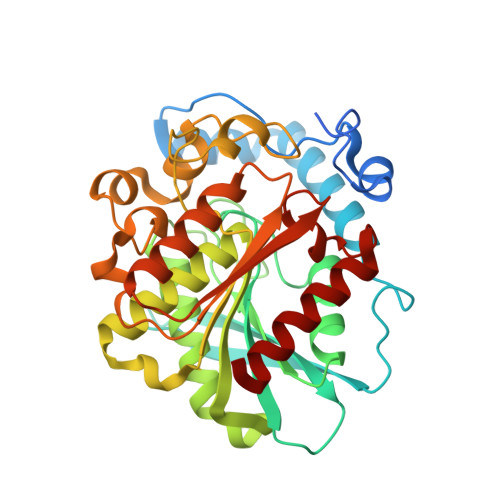
Structural insights of a hormone sensitive lipase homologue Est22.
Huang, J., Huo, Y.Y., Ji, R., Kuang, S., Ji, C., Xu, X.W., Li, J.(2016) Sci Rep 6: 28550-28550
- PubMed: 27328716
- DOI: https://doi.org/10.1038/srep28550
- Primary Citation of Related Structures:
5HC0, 5HC2, 5HC3, 5HC4, 5HC5 - PubMed Abstract:
Hormone sensitive lipase (HSL) catalyzes the hydrolysis of triacylglycerols into fatty acids and glycerol, thus playing key roles in energy homeostasis. However, the application of HSL serving as a pharmaceutical target and an industrial biocatalyst is largely hampered due to the lack of high-resolution structural information. Here we report biochemical properties and crystal structures of a novel HSL homologue esterase Est22 from a deep-sea metagenomic library. Est22 prefers short acyl chain esters and has a very high activity with substrate p-nitrophenyl butyrate. The crystal structures of wild type and mutated Est22 with its product p-nitrophenol are solved with resolutions ranging from 1.4 Å to 2.43 Å. The Est22 exhibits a α/β-hydrolase fold consisting with a catalytic domain and a substrate-recognizing cap domain. Residues Ser188, Asp287, and His317 comprise the catalytic triad in the catalytic domain. The p-nitrophenol molecule occupies the substrate binding pocket and forms hydrogen bonds with adjacent residues Gly108, Gly109, and Gly189. Est22 exhibits a dimeric form in solution, whereas mutants D287A and H317A change to polymeric form, which totally abolished its enzymatic activities. Our study provides insights into the catalytic mechanism of HSL family esterase and facilitates the understanding for further industrial and biotechnological applications of esterases.
Organizational Affiliation:
State Key Laboratory of Genetic Engineering, Collaborative Innovation Center of Genetics and Development, Shanghai Engineering Research Center of Industrial Microorganisms, School of Life Sciences, Fudan University, Shanghai, 200438, China.