Neutralization mechanism of a highly potent antibody against Zika virus
Zhang, S., Kostyuchenko, V.A., Ng, T.-S., Lim, X.-N., Ooi, J.S.G., Lambert, S., Tan, T.Y., Widman, D.G., Shi, J., Baric, R.S., Lok, S.-M.(2016) Nat Commun 7: 13679-13679
- PubMed: 27882950
- DOI: https://doi.org/10.1038/ncomms13679
- Primary Citation of Related Structures:
5H30, 5H32, 5H37 - PubMed Abstract:
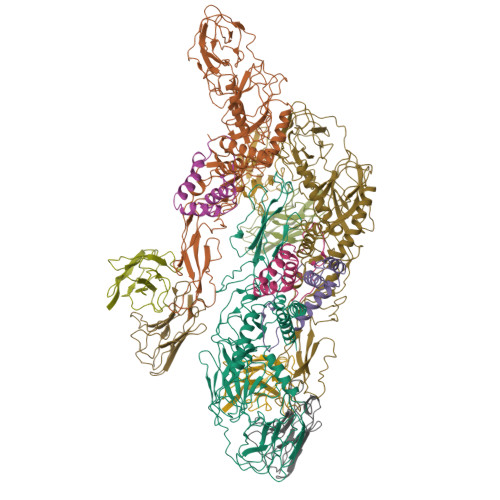
The rapid spread of Zika virus (ZIKV), which causes microcephaly and Guillain-Barré syndrome, signals an urgency to identify therapeutics. Recent efforts to rescreen dengue virus human antibodies for ZIKV cross-neutralization activity showed antibody C10 as one of the most potent. To investigate the ability of the antibody to block fusion, we determined the cryoEM structures of the C10-ZIKV complex at pH levels mimicking the extracellular (pH8.0), early (pH6.5) and late endosomal (pH5.0) environments. The 4.0 Å resolution pH8.0 complex structure shows that the antibody binds to E proteins residues at the intra-dimer interface, and the virus quaternary structure-dependent inter-dimer and inter-raft interfaces. At pH6.5, antibody C10 locks all virus surface E proteins, and at pH5.0, it locks the E protein raft structure, suggesting that it prevents the structural rearrangement of the E proteins during the fusion event-a vital step for infection. This suggests antibody C10 could be a good therapeutic candidate.
Organizational Affiliation:
Program in Emerging Infectious Diseases, Duke-National University of Singapore Medical School, Singapore 169857, Singapore.